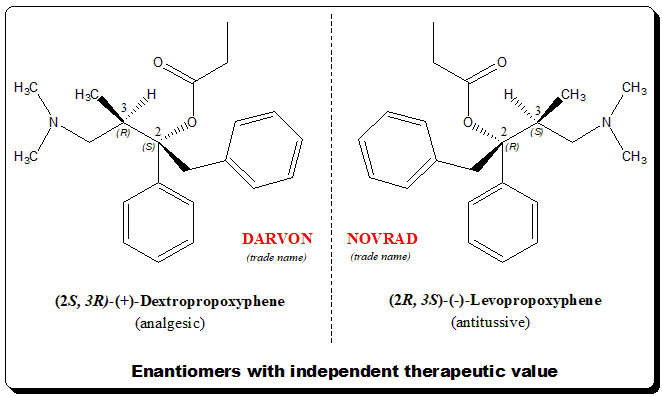
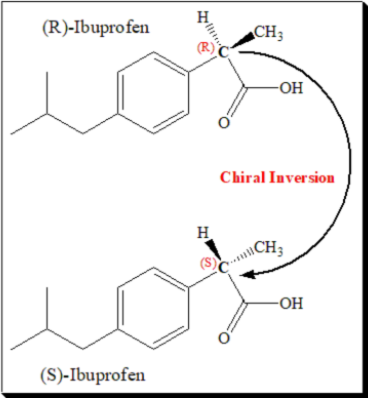
Escitalopram
Citalopram belongs to a class of antidepressant agents known as selective serotonin-reuptake inhibitors (SSRIs). It is indicated for the treatment of depression. Chirality and biological activity Citalopram has one stereocenter, to which a 4-fluoro phenyl group and an N, N-dimethyl-3-aminopropyl group are linked. As a consequence the molecule exists as an enantiomeric pair, the S-(+)-citalopram and R-(–)-citalopram. The selective serotonin-reuptake inhibitor activity resides primarily in the S-enantiomer (eutomer) while the R-enantiomer is 30-fold less potent (distomer). …