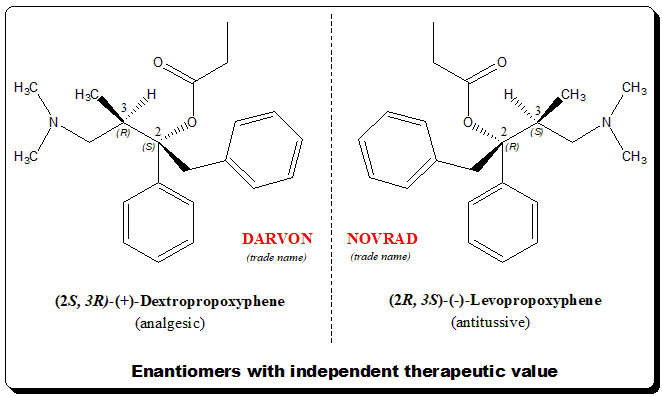
Chiral twins with independent therapeutic value
Propoxyphene is a classical example of a synthetic chiral drug where the enantiomers of a chiral drug exhibit desirable but different therapeutic activity. Example of such biological behavior is exemplified by some of the natural products as well (e.g., quinine and quinidine) with anti-malarial and anti-arrhythmic activity.
Chirality and biological activity
Propoxyphene carries two stereogenic centers meaning it can exist in two pair of enantiomers (4 stereoisomers). One of the enantiomeric pairs the (+)-Dexpropoxyphene is analgesic where as the (-)-Levopropoxyphene is effective antitussive but has no analgesic activity.
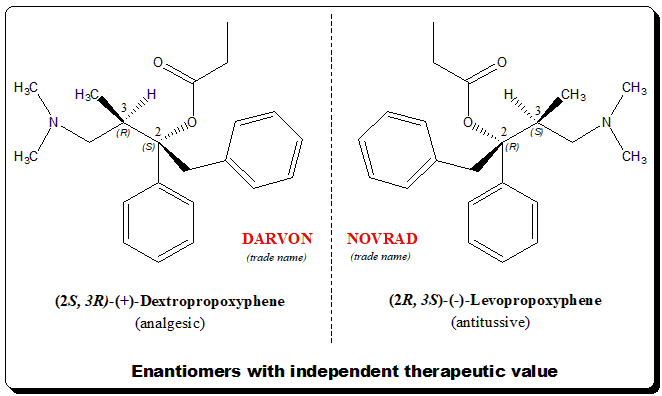
Interesting to note that the mirror-image relationship is reflected in the trade names (DARVON and NOVRAD) under which the drugs are marketed by Eli Lilly.
Nomenclature
Dexpropoxyphene: [(2S,3R)-4-(dimethylamino)-3-methyl-1,2-diphenylbutan-2-yl] propanoate
Levopropoxyphene: [(2R,3S)-4-(dimethylamino)-3-methyl-1,2-diphenylbutan-2-yl] propanoate
Exercise
Understand the nomenclature and stereo-descriptors employed in describing the enantiomeric pair of propoxyphene
References
Chiral drug. Wikipedia, Wikipedia Foundation, 03/08/2022. https://en.wikipedia.org/wiki/Chiral_drugs and references therein
https://pubchem.ncbi.nlm.nih.gov/compound/dextropropoxyphene#section=Names-and-Identifiers
https://pubchem.ncbi.nlm.nih.gov/compound/levopropoxyphene#section=Names-and-Identifiers