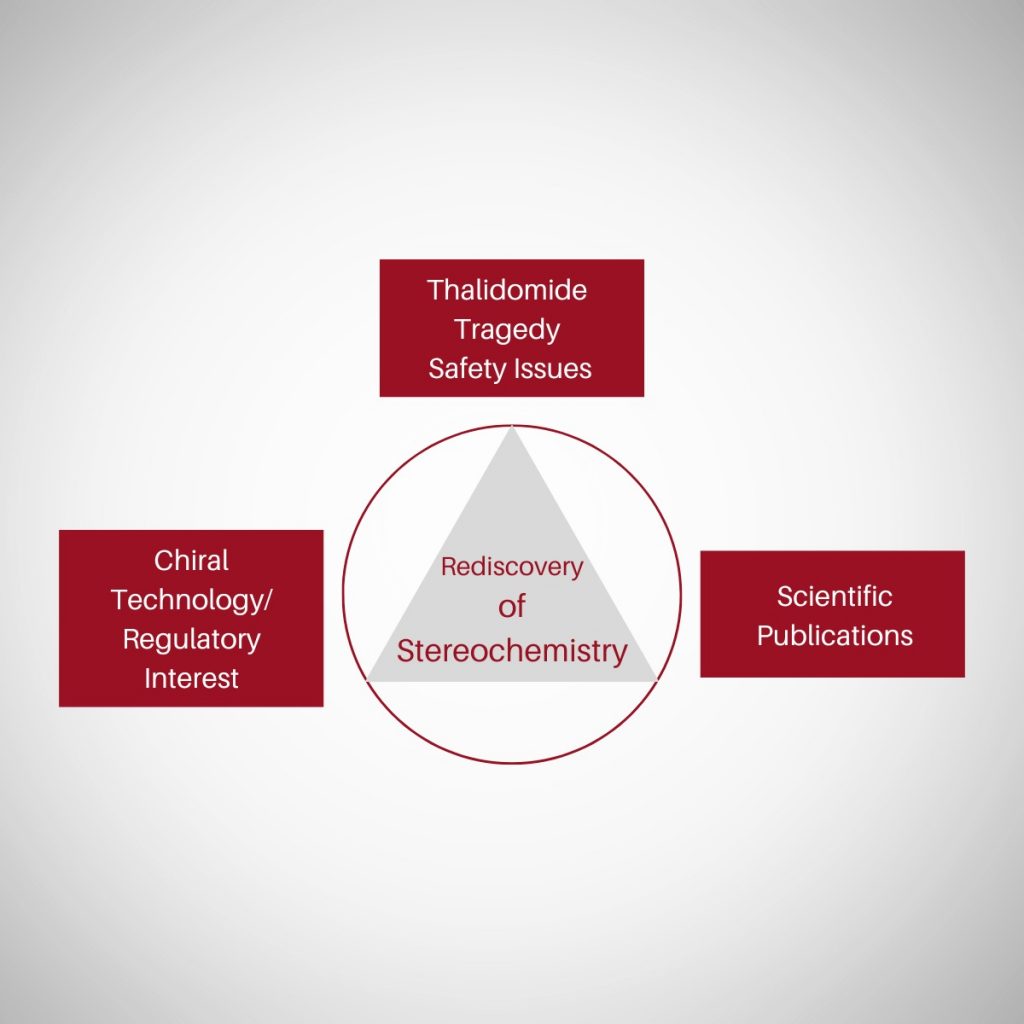
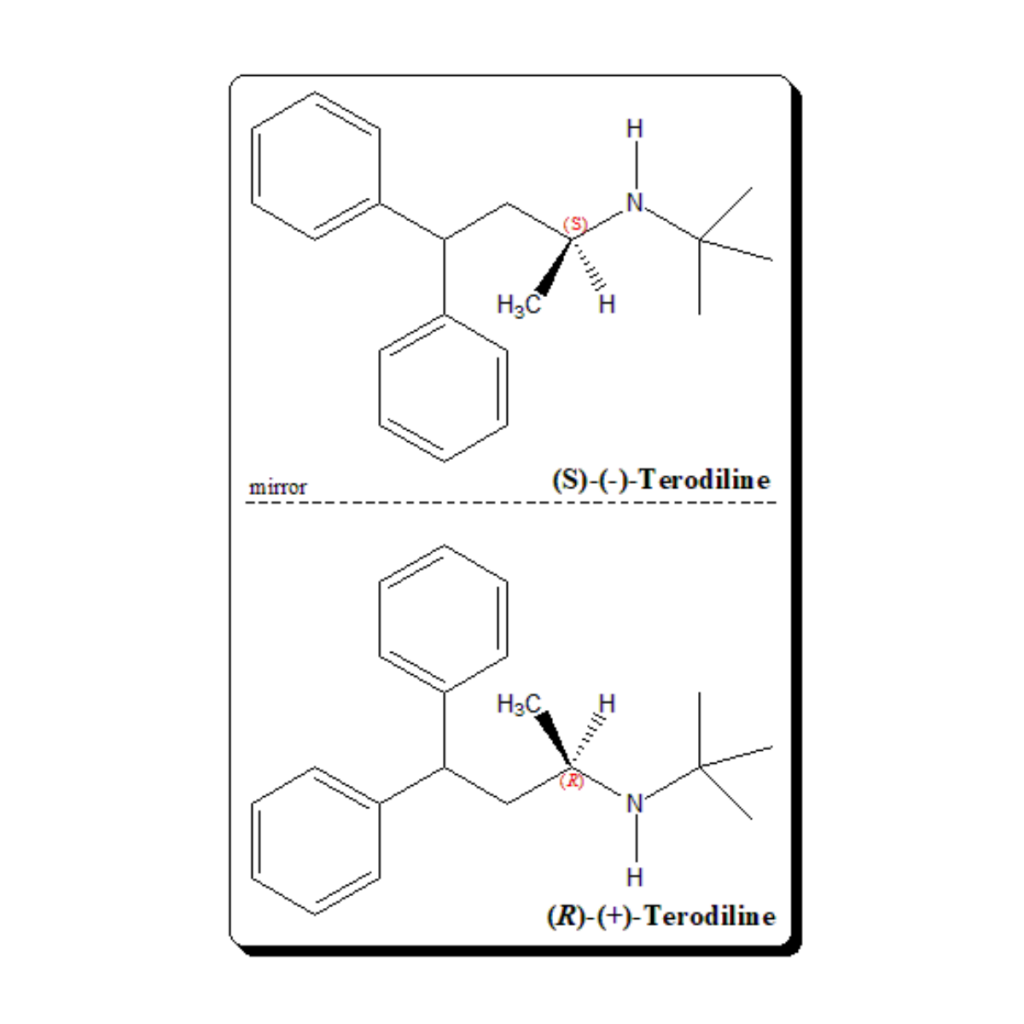
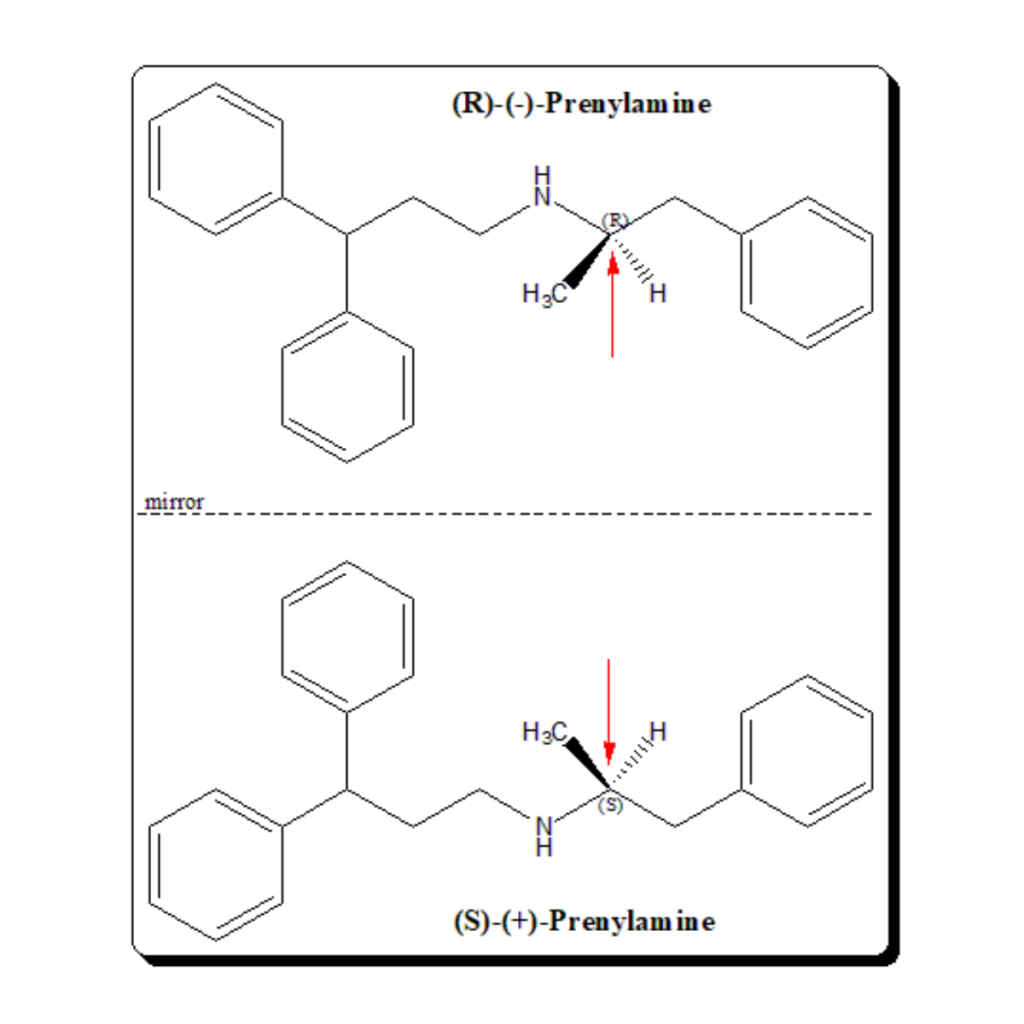
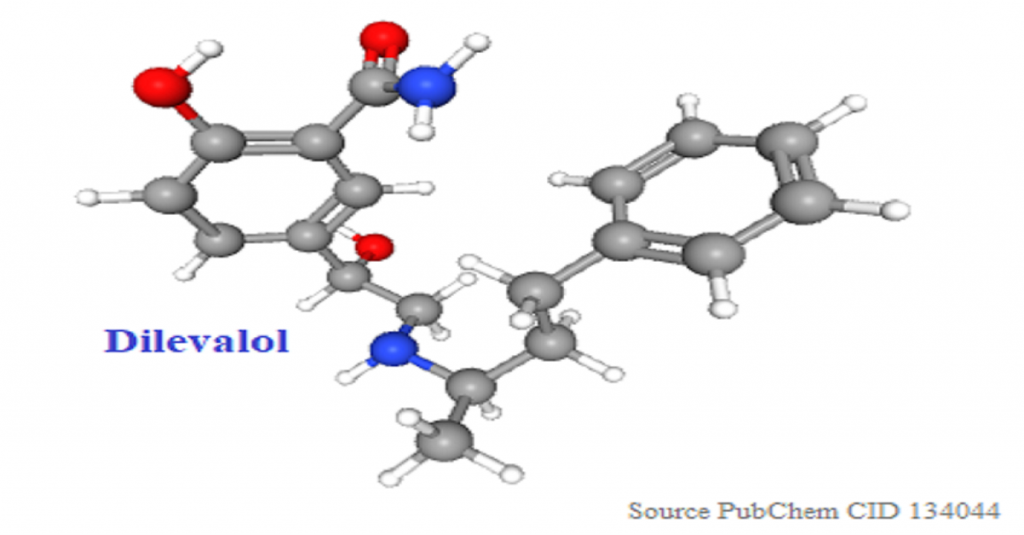
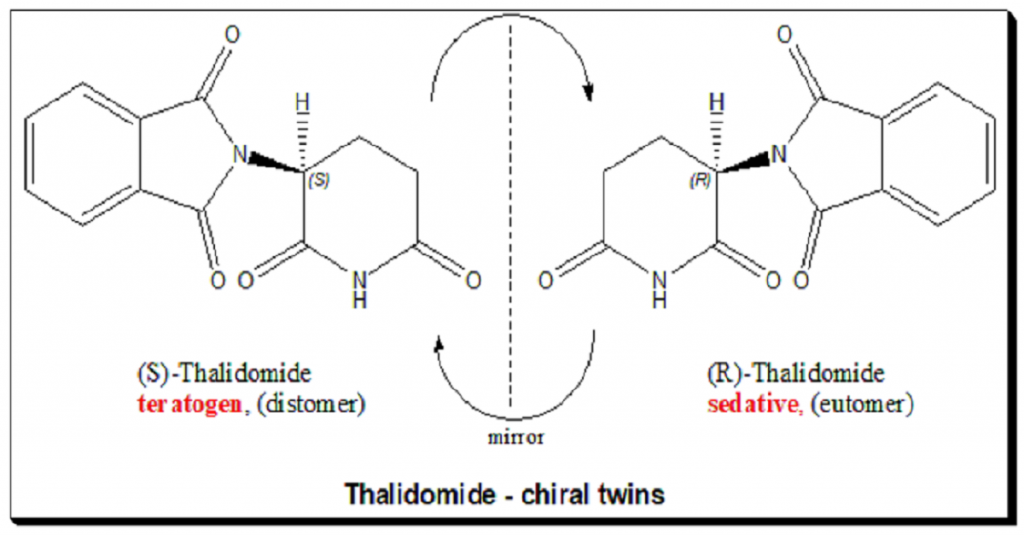
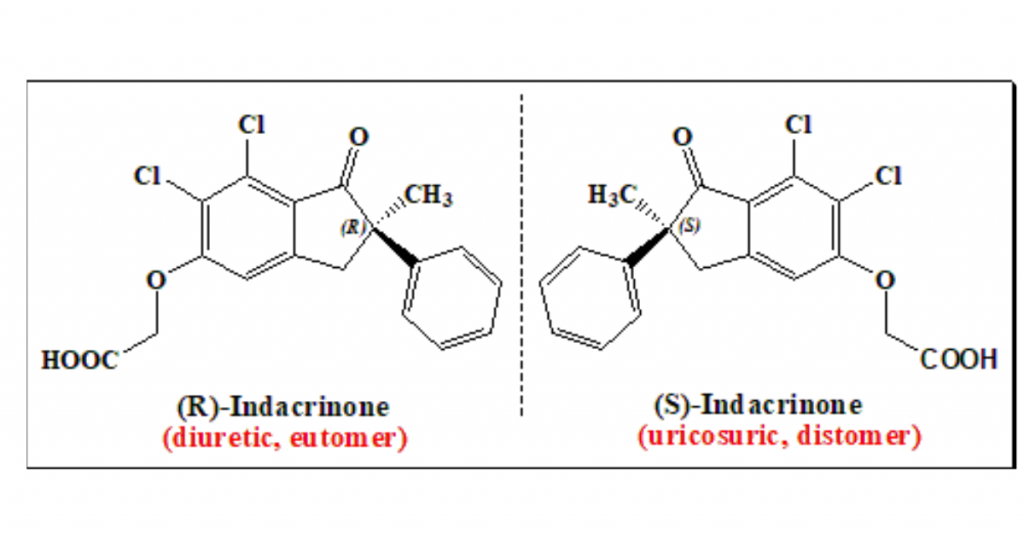
Rediscovery of stereochemistry
Stereochemistry, in particular chirality, is known to us as early as 1809, when Malus, Arago and Biot discovered plane polarized light and its characteristics. It is observed that during 1950s to the 1970s, the “Golden Age” of drug discovery & development, stereochemistry was largely ignored resulting in approximately 57% of pharmaceuticals being marketed as racemates by the 1980s. Before going further let us examine the stereospecific awareness level that existed during 1980s’. Stereospecific Awareness level …