
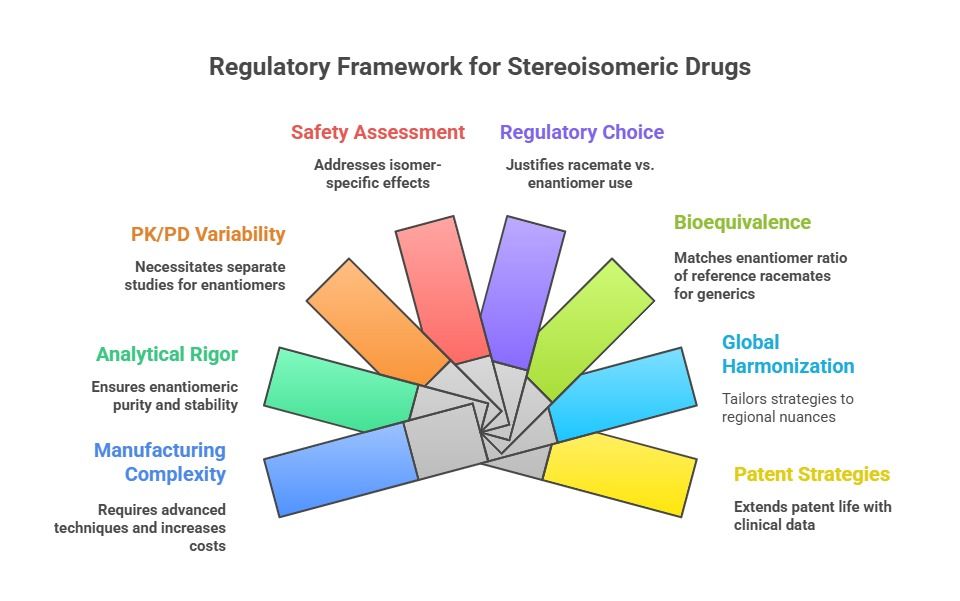
Stereoisomerism, where molecules share the same structure but differ in 3D orientation, profoundly impacts drug efficacy and safety. Enantiomers (mirror-image isomers) and diastereomers (non-mirror-image) can exhibit distinct pharmacological profiles. For instance, while one enantiomer may be therapeutic, another could be inert or toxic, as seen in thalidomide's tragic legacy. This complexity necessitates rigorous regulatory scrutiny to ensure patient safety and therapeutic efficacy.
The thalidomide disaster (1950s–60s), where a racemic mixture caused birth defects, underscored the need for stereochemical consideration in drug approvals. Although thalidomide's enantiomers interconvert in vivo, the incident catalyzed regulatory reforms. Landmark guidelines, such as the FDA's 1992 Policy on Stereoisomeric Drugs and EMA's reflection papers, now mandate thorough stereoisomer characterization. The International Council for Harmonisation (ICH) further harmonizes standards via guidelines like Q6A, emphasizing enantioselective analysis.
Today, global regulatory authorities such as the FDA, EMA, PMDA, and others enforce rigorous standards for the development, characterization, and approval of stereoisomeric drugs. Developers must demonstrate not only the activity of each isomer but also justify decisions related to the use of single enantiomers versus racemates. Regulatory guidelines demand comprehensive data on stereoisomeric stability, interconversion, pharmacokinetics, pharmacodynamics, and toxicological profiles.