The chiral switches of ‘profen’ NSAIDs
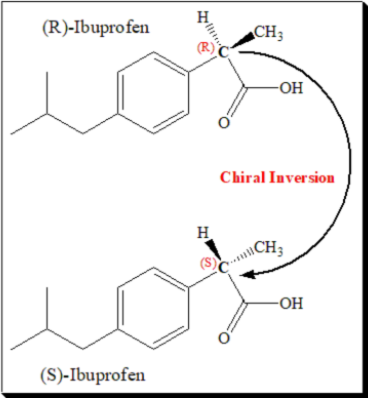
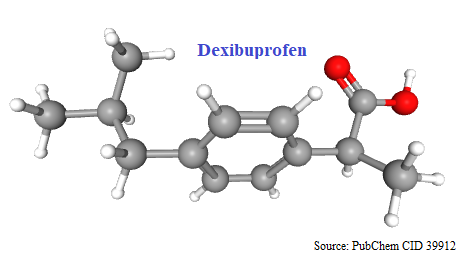
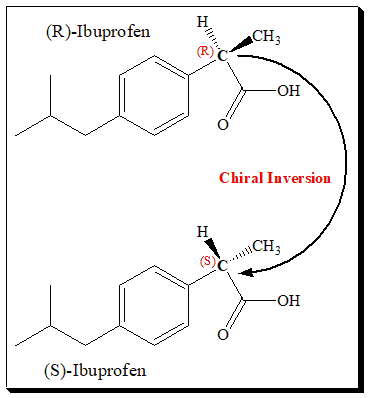
Chiral switches have been successful in two non-steroidal anti-inflammatory drugs (NSAIDs) – ibuprofen and ketoprofen. The switch of ibuprofen is based on chiral inversion; (±)- and (S)-ibuprofen can be viewed as being essentially bioequivalent’. Ibuprofen carries a stereogenic center in the propanoic acid side chain and exists as a pair of enantiomers. Dexibuprofen is (S)-(+)-Ibuprofen.
Chiral Inversion
COX inhibition resides exclusively in the (S)-enantiomer, as shown by in vitro studies in a wide range of test systems. However, the activities of the two enantiomers of many profens are essentially indistinguishable in vivo, due to the unidirectional metabolic bioconversion of the (R)-enantiomer to the (S)-enantiomer, referred to as chiral inversion. The extent of this inversion differs between compounds and species, and in some cases, the inactive (R)-enantiomers are pro-drugs for the active (S)-forms.
Nomenclature
(2S)-2-[4-(2-methylpropyl)phenyl]propanoic acid
Exercise
Understand the phenomenon of chiral inversion
References
Israel Agranat, Hava Caner and John Caldwell, Nature reviews, Drug Discovery, 1, October,| 753-768, 2002.
Chiral switch. Wikipedia, Wikipedia Foundation, 201/08/2022. https://en.wikipedia.org/wiki/Chiral_switch
Lien Ai Nguyen, Hua He, and Chuong Pham-Huy, Chiral drugs: An overview, Int J. Biomed. Sci. 1,2,85-100, 2006; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3614593/