Citalopram belongs to a class of antidepressant agents known as selective serotonin-reuptake inhibitors (SSRIs). It is indicated for the treatment of depression.
Chirality and biological activity
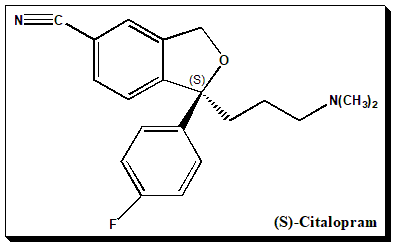
Citalopram has one stereocenter, to which a 4-fluoro phenyl group and an N, N-dimethyl-3-aminopropyl group are linked. As a consequence the molecule exists as an enantiomeric pair, the S-(+)-citalopram and R-(–)-citalopram.
The selective serotonin-reuptake inhibitor activity resides primarily in the S-enantiomer (eutomer) while the R-enantiomer is 30-fold less potent (distomer). To know more about eutomer and distomer nomenclature read @ <https://chiralpedia.com/blog/chiral-twins-identical-but-not-really/> In clinical trials, both racemic (R,S)-citalopram and (S)-citalopram were significantly better than placebo for improving depression and data suggests that (S)-citalopram has greater efficacy than and fewer side-effects than the (±)-citalopram. By and large, (S)-citalopram appears to have advantages over (±)-citalopram and is a good example of the potential benefits of enantiopure drugs.
Reasoning for the chiral switch
- Faster onset of action
- Reduction in side effects and tolerability profile
- S-enantiomer has greater potency in comparison to the R- enantiomer in the inhibition of 5-HT uptake, eudisimic ratios (S/R) between 130 and 160 depending on test system used. To know about eudisimic ratio, read more @ my page in Wikipedia – Chiral drugs. Wikipedia, Wikipedia Foundation, 10/09/2022. https://en.wikipedia.org/wiki/Chiral_drugs>.
- Better risk-benefit ratio compared to R-Citalopram
Nomenclature
(1S)-1-[3-(dimethylamino)propyl]-1-(3-fluorophenyl)-3H-2-benzofuran-5-carbonitrile
Exercise
Understand the terms eutomer, distomer and eudisimic ratio
Comprehend numbering of the heterocyclic ring system and the nomenclature of escitalopram
Reference
https://pubchem.ncbi.nlm.nih.gov/compound/Escitalopram
Chiral switch. Wikipedia, Wikipedia Foundation, 08/08/2022. https://en.wikipedia.org/wiki/Chiral_switch and references therein
Jonathan McConathy, and Michael J. Owens. Stereochemistry in Drug Action, Prim Care Companion J. Clin. Psychiatry.5(2): 70–73, 2003. DOI: 10.4088/pcc.v05n0202
C. Lindsay DeVane and David W. Boulton. Great Expectations in Stereochemistry: Focus on Antidepressants, CNS Spectrums , Volume 7 , Issue S1: The Biological and Clinical Significance of Single Isomers , 28 – 33, 2002. DOI: https://doi.org/10.1017/S1092852900028571
Harkishan Singh and V.K. Kapoor. Medicinal chemistry and pharmaceutical chemistry, Vallabh Prakashan, New Delhi, Page 163-164, 2012.
Chiral drugs. Wikipedia, Wikipedia Foundation, 10/09/2022. https://en.wikipedia.org/wiki/Chiral_drugs and references therein
https://chiralpedia.com/blog/chiral-twins-identical-but-not-really/