Synopsis
The development of chromatographic methods for the separation of chiral drugs presents a significant challenge, necessitating a comprehensive understanding of the compounds’ chemical properties and the factors influencing their separation. This task is further complicated by the presence of process-related impurities that can disrupt the separation process and compromise the quality of the results. However, the application of advanced statistical tools such as JMP’s Design of Experiments (DoE) and Prediction Profiler can significantly streamline this process and optimize the chromatographic conditions.
Introduction
Enantiomers, molecular mirror-images of each other, exhibit drastically different interactions with other molecules, leading to varying biological effects. This characteristic necessitates the separation of enantiomers in the pharmaceutical industry, where one enantiomer of a drug may have the desired therapeutic effect, while the other may be inactive or even harmful. Chromatography, a technique that separates compounds based on their interaction with a stationary phase and mobile phase, is typically used for this separation. However, the separation of enantiomers presents unique challenges due to their similar physical and chemical properties. The presence of process-related impurities can further complicate the separation process, making it difficult to achieve the desired resolution and purity.
The Role of JMP’s DoE and Prediction Profiler
JMP’s DoE and Prediction Profiler are potent statistical tools that can help overcome these challenges. DoE allows for the systematic variation of experimental factors to understand their impact on the response, while the Prediction Profiler provides a visual representation of the relationship between the factors and the response, facilitating the optimization process.
Case Studies and Applications
Case Study 1: Optimization and Validation of a RP-HPLC Method for the Enantioselective Analysis of Ofloxacin in Pharmaceutical Formulations (for a detailed discussion read Ref 1.)
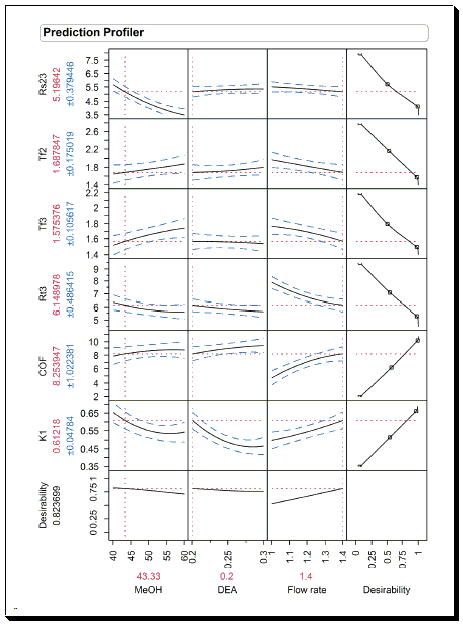
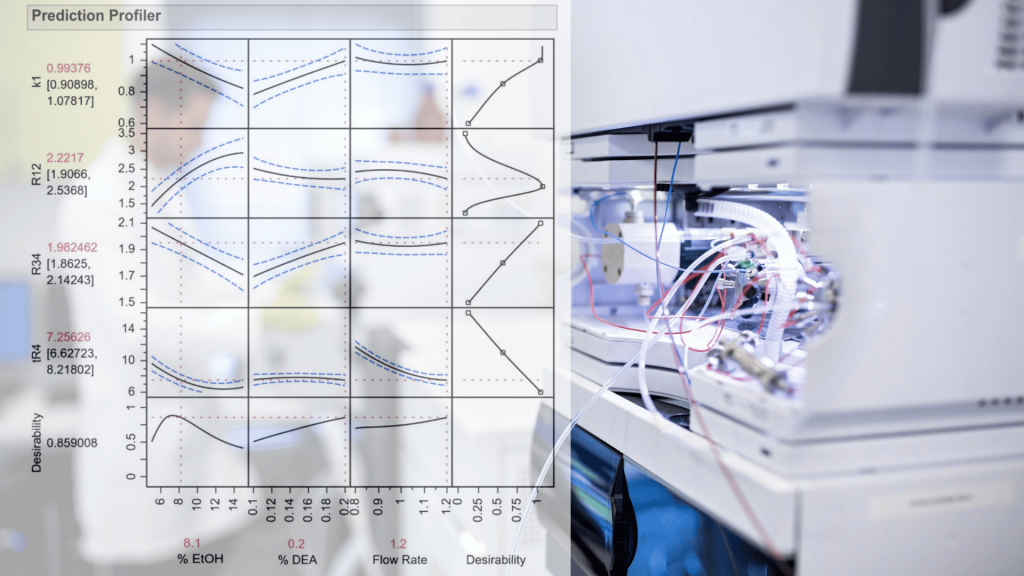
In this case, the JMP prediction profiler was used to optimize the separation of the Ofloxacin enantiomeric pair. The goal was to minimize the resolution between the Ofloxacin enantiomeric pair, reduce the tailing factors of enantiomers, minimize the analysis time, increase the COF value, and the retention factor k1.The highest desirability value of 0.823 was achieved at MeOH/hexane/MeCn-43.33/10/46.62 (v/v) %, % AcOH/DEA-0.4/0.2, and flow rate as 1.4 ml min−1. The predicted response values corresponding to the highest desirability value (0.823) were k1 = 0.61, Rs23 = 5.193, tF2 = 1.68, tF3 = 1.57, tR3 = 6.14 min, and COF = 8.25.
Case Study 2: Simultaneous Enantioseparation and Purity Determination of Chiral Switches of Amlodipine and Atenolol by Liquid Chromatography (for a detailed discussion read Ref 2.)
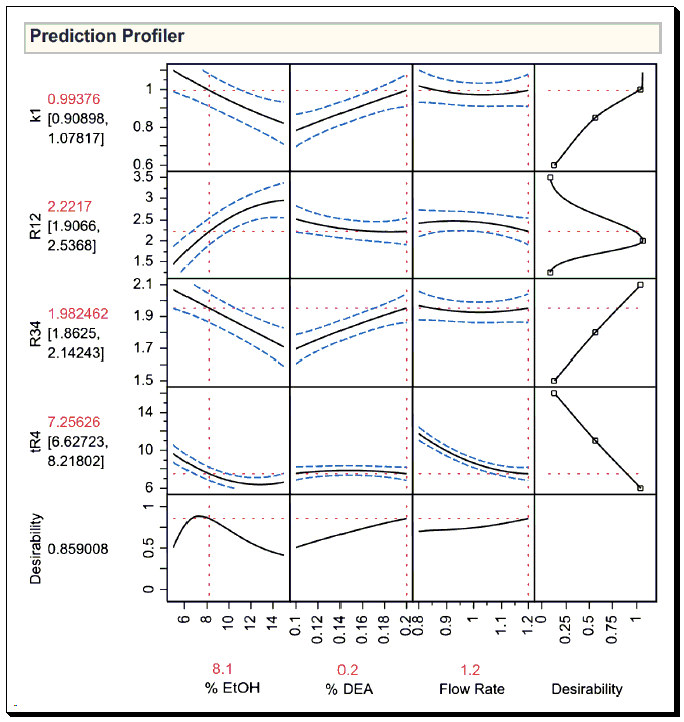
In this instance, the JMP prediction profiler was used to optimize the separation of chiral molecules in high-performance liquid chromatography (HPLC). The document discusses the application of the JMP prediction profiler in optimizing the separation of chiral molecules in chromatography. The JMP prediction profiler is used to visualize the relationship between the factors (like mobile phase, additives, temperature and flow rate) and the response (capacity factor, resolution, chromatographic run time). In the case study presented, the goal was to maximize the resolution of separation of a pair of chiral molecules. The JMP prediction profiler was used to adjust the factors and observe the effect on the resolution. By doing this, we were able to find the optimal conditions that provided the best separation of the pair of chiral molecules. This approach allows for a more efficient and effective optimization process in chromatographic separation.
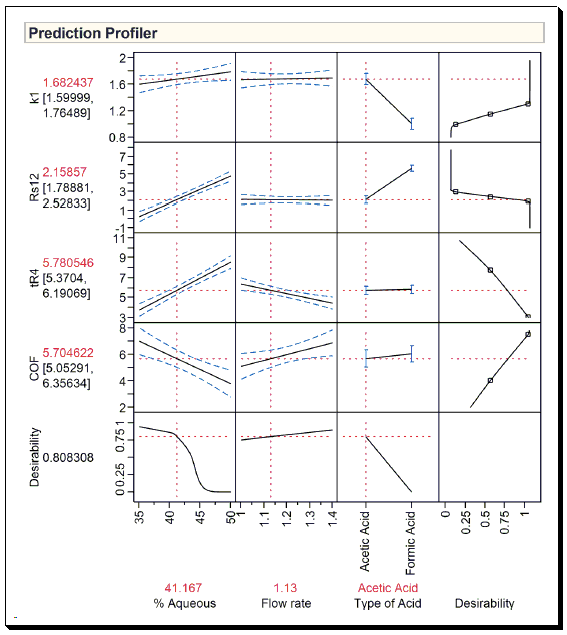
Case Study 3: Optimization and Robustness Testing of a RP-HPLC Method for Determination of Warfarin and its Process-Related Impurities (for a detailed discussion read Ref 3.)
In this case, the JMP prediction profiler was used to optimize the separation of the Ofloxacin enantiomeric pair. The goal was to minimize the resolution between the Ofloxacin enantiomeric pair, reduce the tailing factors of enantiomers, minimize the analysis time, increase the COF value, and the retention factor k1. The highest desirability value of 0.823 was achieved at MeOH/hexane/MeCn-43.33/10/46.62 (v/v) %, % AcOH/DEA-0.4/0.2, and flow rate as 1.4 ml min−1. The predicted response values corresponding to the highest desirability value (0.823) were k1 = 0.61, Rs23 = 5.193, tF2 = 1.68, tF3 = 1.57, tR3 = 6.14 min, and COF = 8.25.
Conclusion
The use of JMP’s DoE and Prediction Profiler in the development of chromatographic methods for the separation of chiral drugs offers a robust and efficient approach to overcome the challenges associated with this process. By providing a visual representation of the relationship between the factors and the response, these tools facilitate the optimization process, allowing for the identification of the optimal conditions that provide the best separation of the chiral molecules. This not only improves the efficiency of the separation process but also enhances the quality of the results, making it a valuable tool in the pharmaceutical industry.
References
- Valliappan Kannappan and Sai Sandeep Mannemala (2014). Multiple response optimization of a HPLC method for the determination of enantiomeric purity of S-Ofloxacin. Chromatographia, 77, 1203-1211. https://doi.org/10.1007/s10337-014-2699-4
- Valliappan Kannappan and Sai Sandeep Mannemala (2016). Simultaneous enantioseparation and purity determination of chiral switches of amlodipine and atenolol by liquid chromatography. Journal of Pharmaceutical and Biomedical Analysis, 120, 221-227. https://doi.org/10.1016/j.jpba.2015.12.048
- Sai Sandeep Mannemala and Valliappan Kannappan (2015). Statistical design in optimization and robustness testing of a RP-HPLC method for determination of Warfarin and its process related impurities. J IRAN CHEM SOC., 12, 1325–1332. https://doi.org/10.1007/s10337-012-2373-7