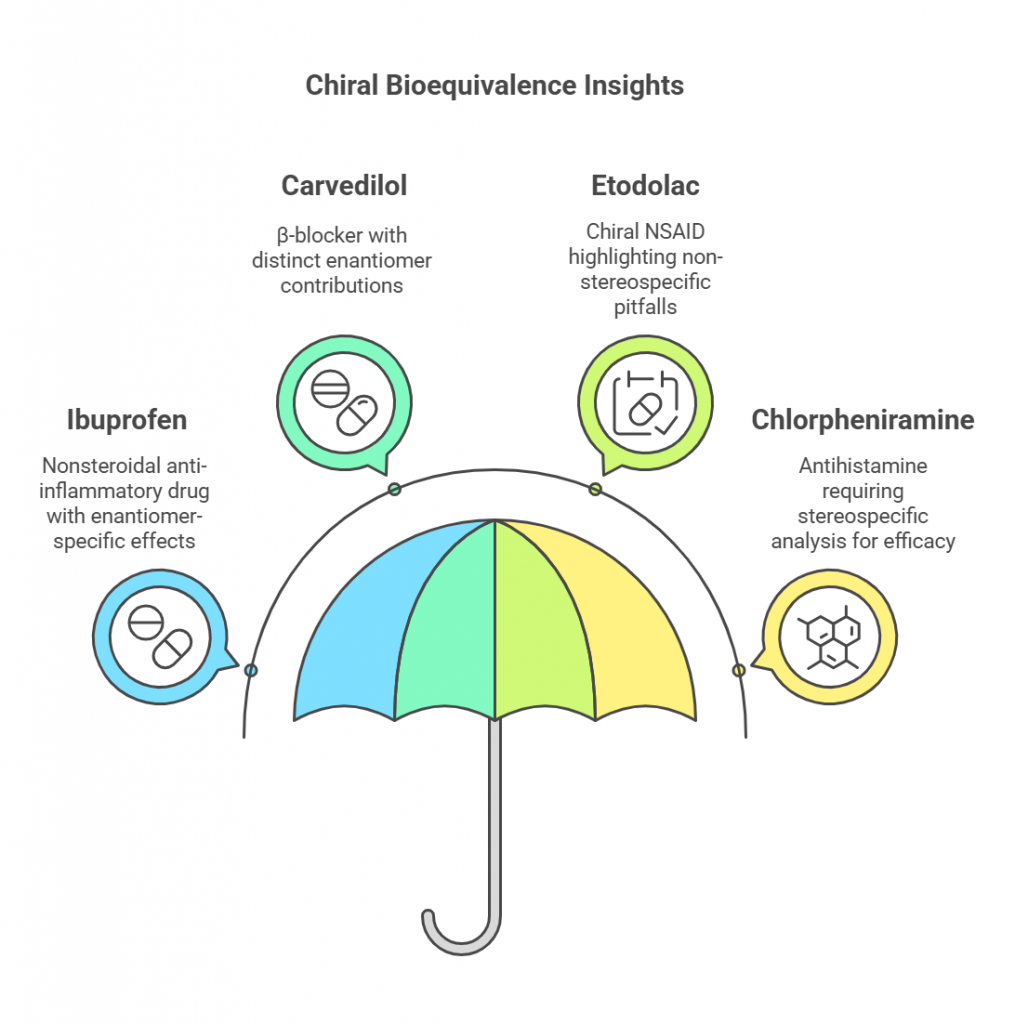
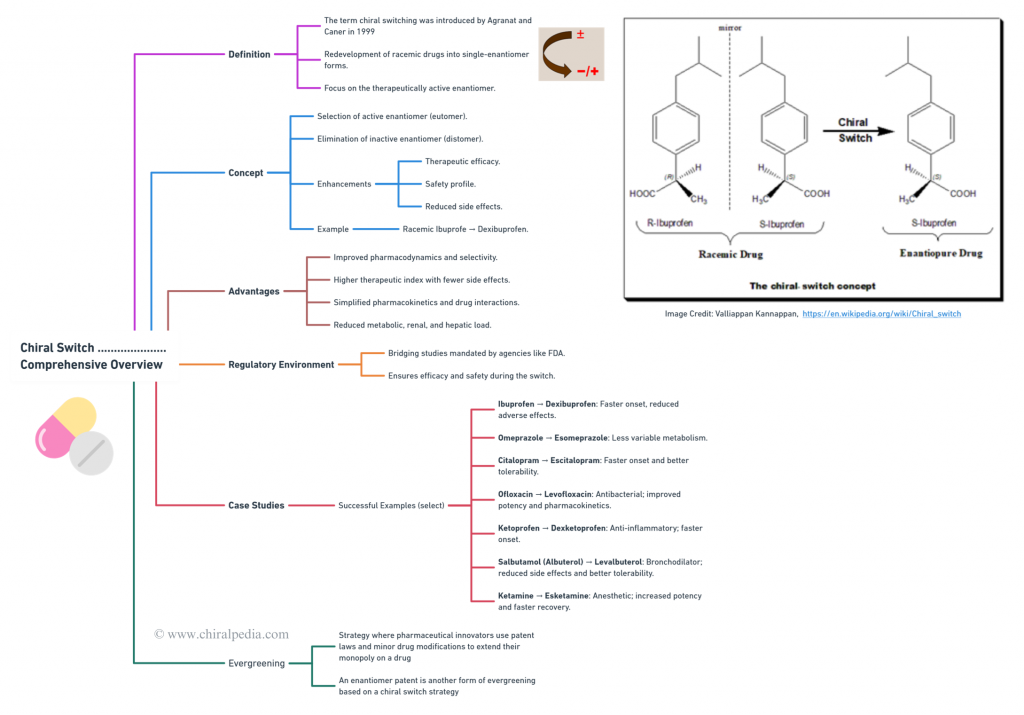
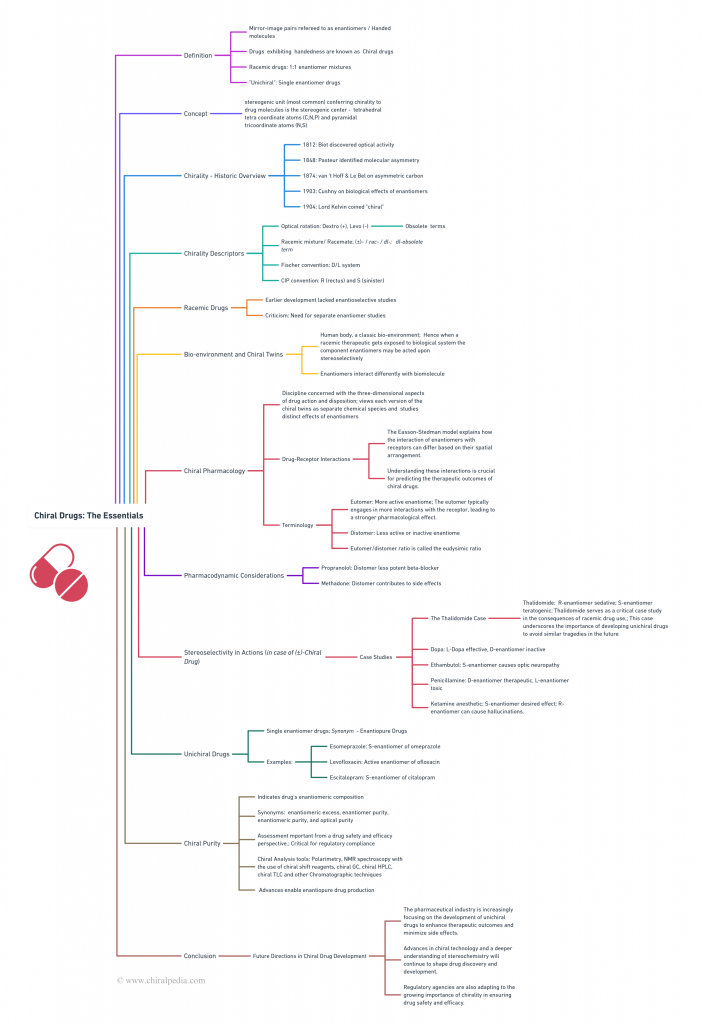
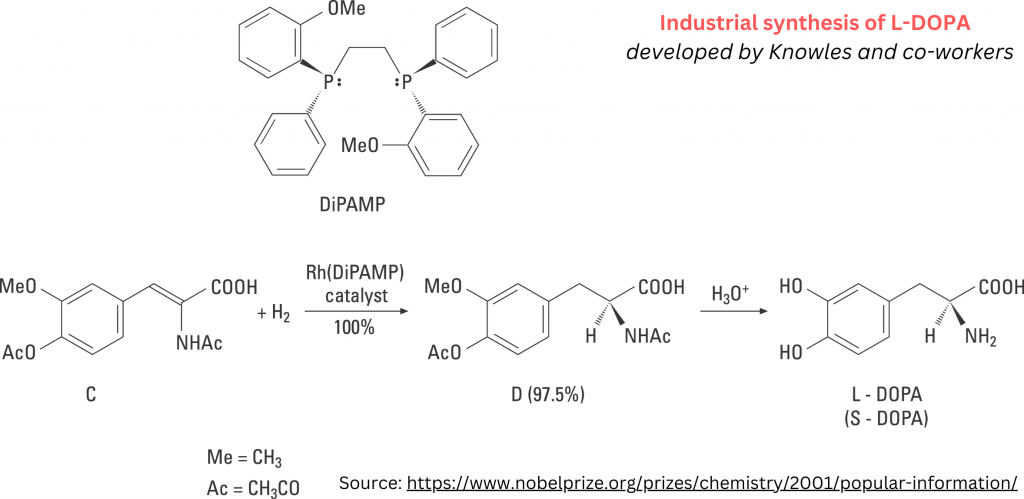
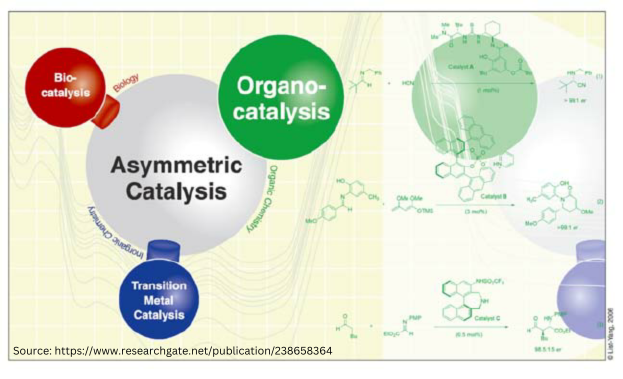
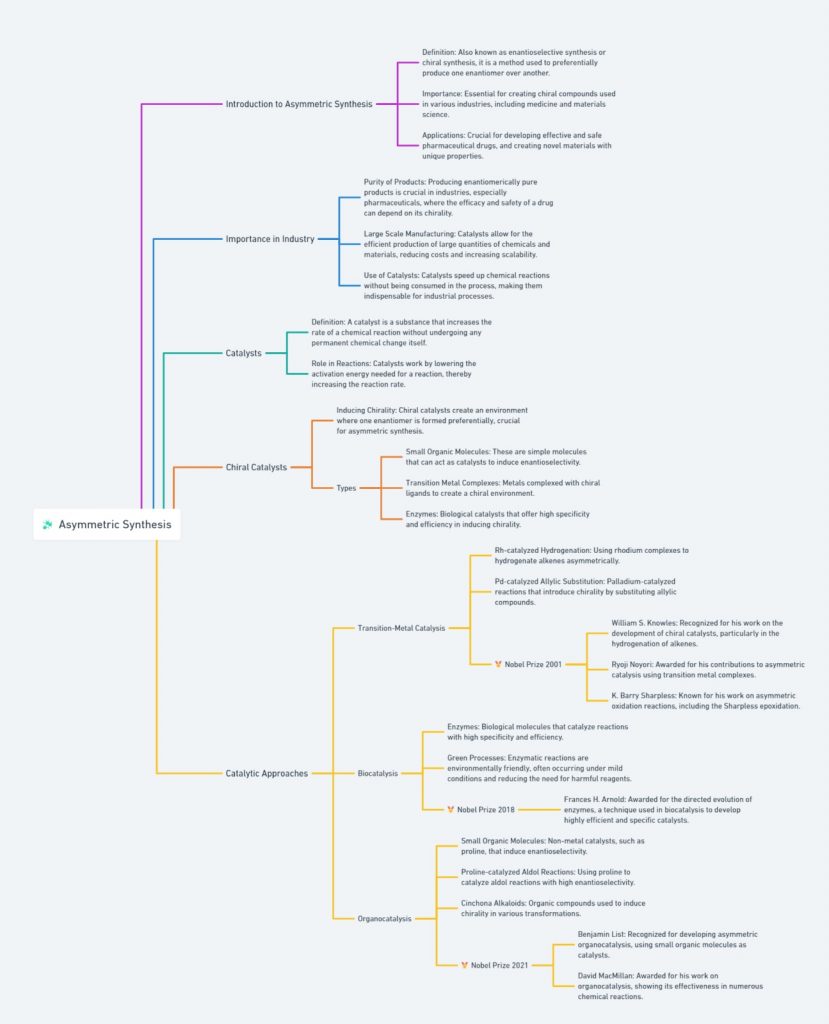
Chiral Bioequivalence – An Explainer
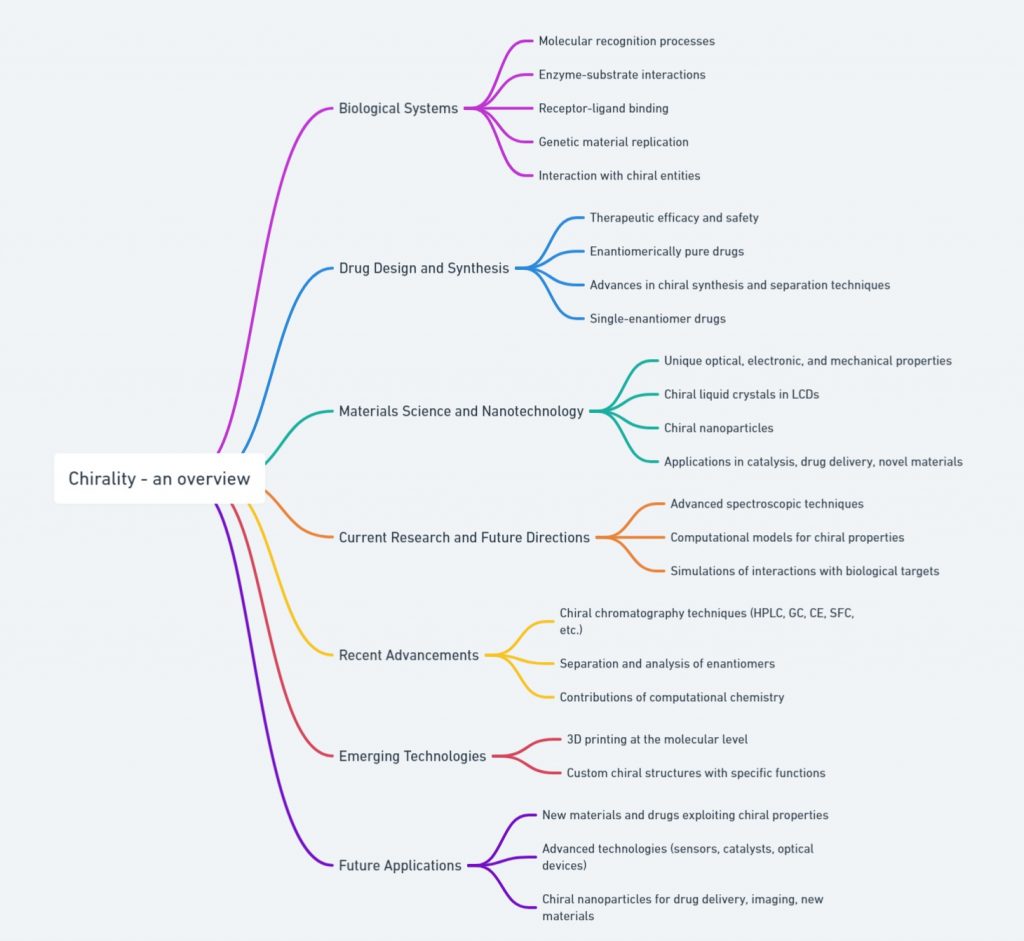
Introduction Chirality, or “handedness,” is the unique property of molecules that exist as non-superimposable mirror images, known as enantiomers. Drugs that exhibit handedness are referred to as chiral drugs. Chiral medications consisting of an equal (1:1) mixture of enantiomers are called Racemic drugs. In drug development, chirality is incredibly important because most biological molecules, like enzymes and receptors, are chiral. This characteristic affects how a drug interacts with biological targets, leading to significant differences in …