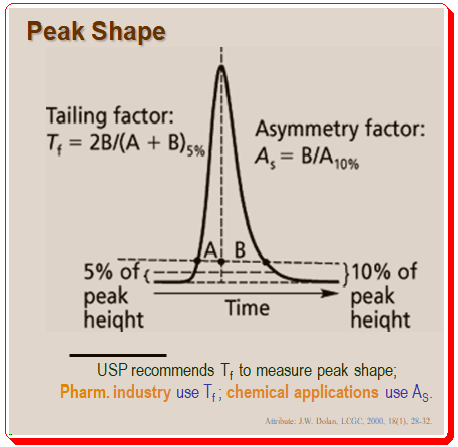
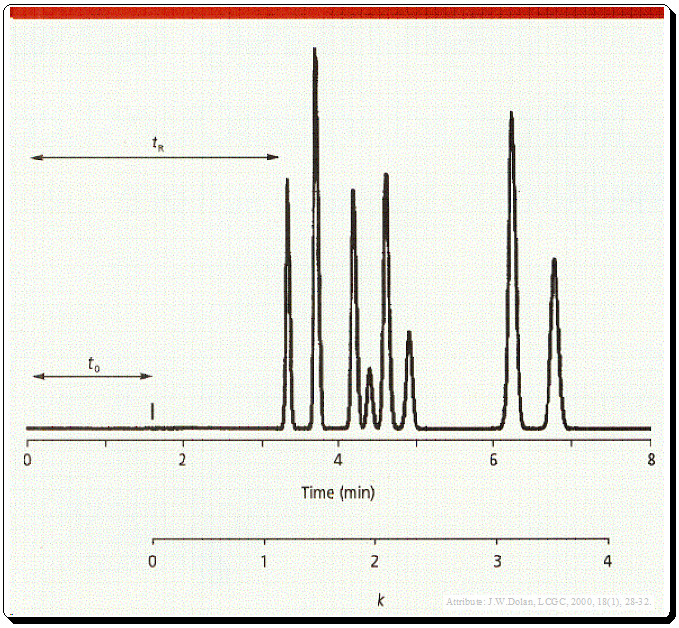
Introduction to Chirality: Understanding the Basics
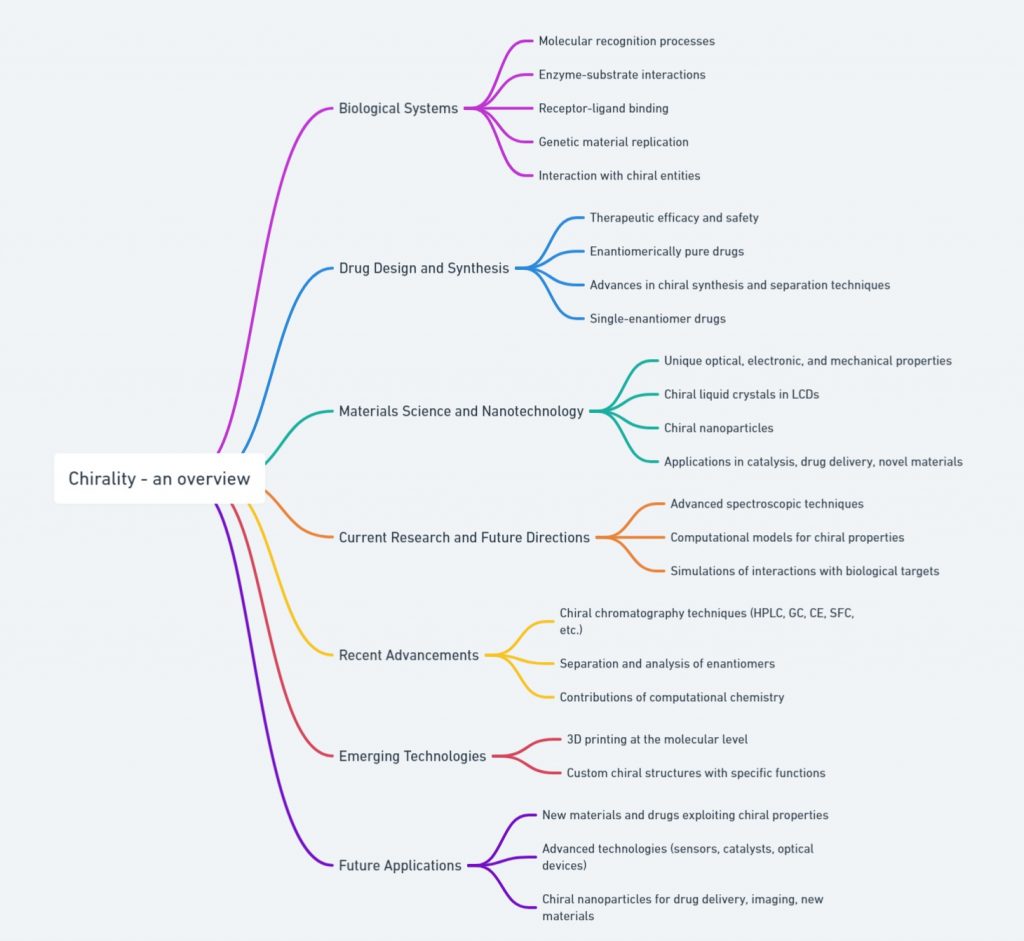
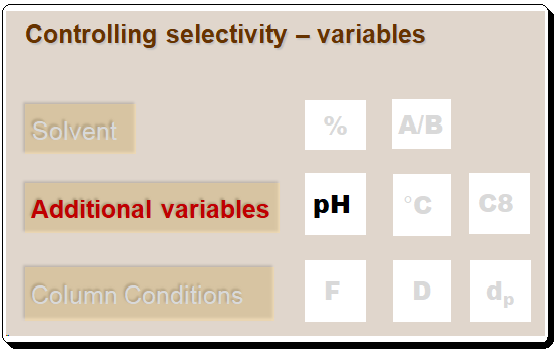
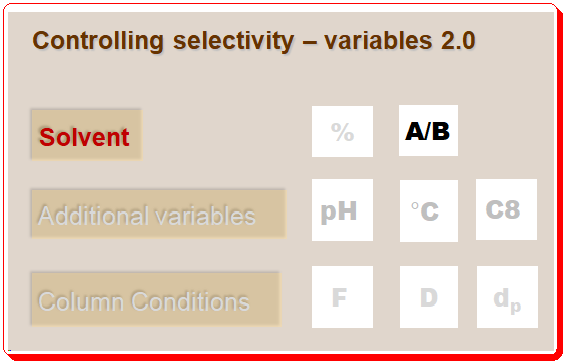
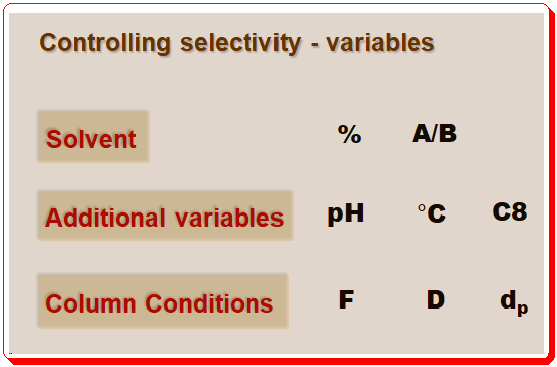
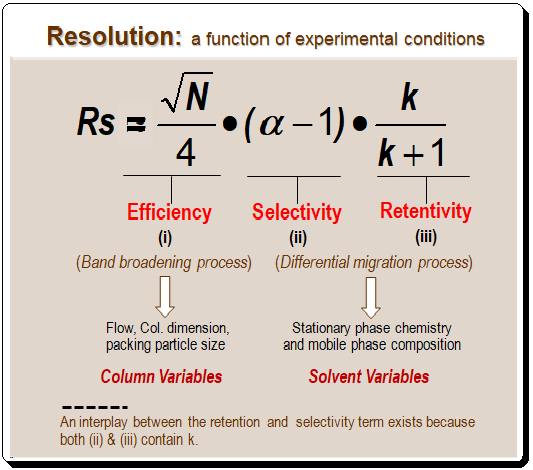
Lead Chirality, derived from the Greek word “cheir” meaning hand, is a fundamental concept in chemistry that describes an object’s property of being non-superimposable on its mirror image. This property is not just an abstract mathematical idea but has profound implications in various scientific fields, particularly chemistry and biology. Understanding chirality is essential for comprehending molecular interactions and their impacts on our daily lives. This first of this blog series delves into the definition, historical …
Introduction to Chirality: Understanding the Basics Read More »