
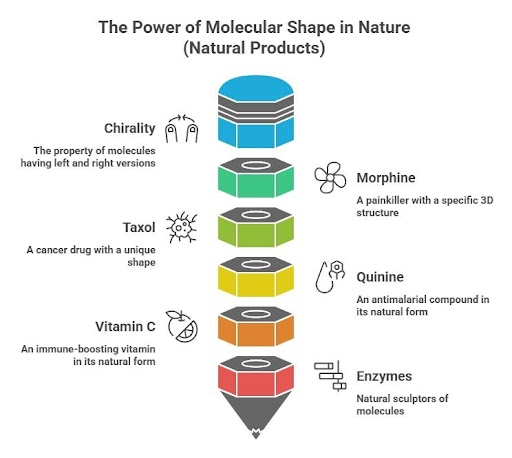
Did you know that molecules, like your hands, can have a “left” and “right” version? This idea, called chirality, is a key part of how nature works. In medicines, scents, and even poisons, the 3D shape of a molecule—how its atoms are arranged—can decide whether it heals or harms.
Take morphine, the famous painkiller from poppy plants. It has five special twists in its structure, allowing it to fit perfectly into the body's pain-relief receptors—like a key in the right lock. Out of 32 possible versions, only one works: the exact one made by nature. The same is true for Taxol, a cancer drug that stops tumor cells from growing. Its unique 3D shape lets it target cancer in a way no other version can. And in everyday health, quinine fights malaria, while vitamin C keeps our immune system strong—but only in their natural forms.
Nature has been designing these powerful molecules for millions of years, using enzymes like tiny sculptors to build the perfect version every time. It's a reminder that in biology, shape isn't just important—it's everything.
Ultimately, studying chirality bridges the gap between biology and chemistry, revealing how life’s molecules work and guiding us toward smarter, safer medicines. It’s a reminder that in nature—and science—the smallest details often matter most.