Understanding the Fundamentals of Asymmetric Synthesis
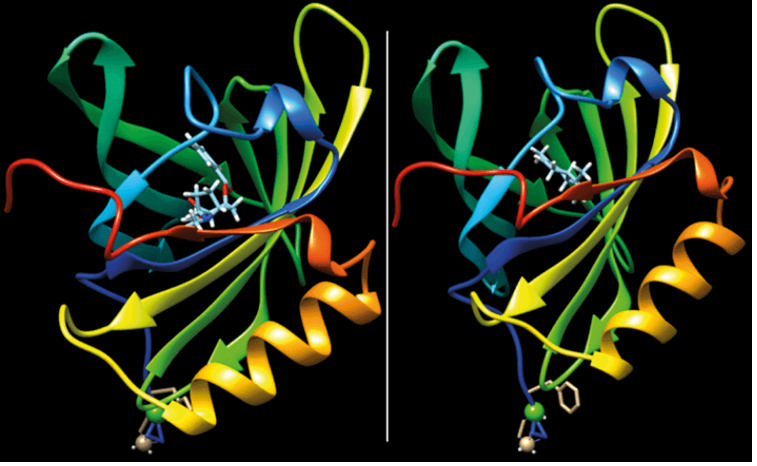
Introduction Chirality is a fundamental concept in chemistry that plays a crucial role in the structure and function of molecules. Derived from the Greek word for “hand,” chirality refers to the geometric property of a molecule that makes it non-superimposable on its mirror image. This characteristic is significant in various fields, including pharmaceuticals, where the spatial arrangement of atoms within a molecule can drastically affect its biological activity. Basic Concepts Stereoisomerism and Chirality Chirality is …
Understanding the Fundamentals of Asymmetric Synthesis Read More »