
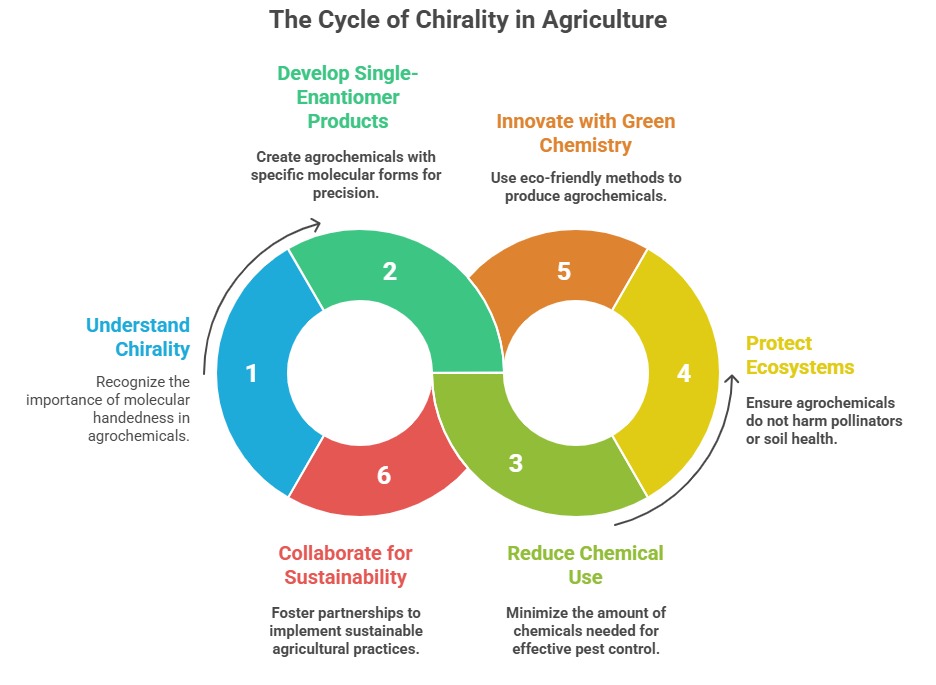
Just as a left-handed glove won’t fit a right hand, many agrochemicals—herbicides, pesticides, fungicides—rely on their molecular “handedness” (chirality) to work. A molecule and its mirror image (enantiomers) may look identical, but their impacts can diverge wildly. One might kill a pest; the other could harm pollinators or linger in soil. For example, the R-form of the fungicide metalaxyl is 1,000x more effective than its mirror image. This has driven a shift toward “single-enantiomer” agrochemicals—products fine-tuned to hit targets precisely, cut chemical use, and protect ecosystems.
Nature isn’t symmetrical, and neither are the challenges farmers face. Racemic mixtures (containing both enantiomers) waste resources: half the formula may be useless or risky. Inactive enantiomers can degrade into toxins or accumulate unseen, threatening soil health and biodiversity. Modern tools like chiral chromatography now let scientists separate and study these mirror-image molecules, guiding regulations to demand cleaner, smarter solutions.
Innovations are already sprouting. Metalaxyl-M, a pure R-enantiomer fungicide, slashes application rates in half. Green chemistry methods, from enzyme-driven synthesis to solvent-free processes, are making production cleaner and cheaper. The next frontier? AI could design chiral agrochemicals tailored to resist rainwash, target pests more narrowly, or break down harmlessly after use.
Chirality isn’t just chemistry—it’s a blueprint for sustainable farming. By embracing single-enantiomer solutions, agriculture can boost yields while shrinking its chemical footprint. But success hinges on collaboration: chemists, ecologists, and policymakers must unite to scale these advances, balance costs, and safeguard ecosystems.