(±)-Prenylamine, an antianginal agent, was introduced in the market since early 1960. Reports associating Prenylamine with prolongation of the QT interval, ventricular tachycardia, and ventricular fibrillation started to appear. Some of these events had a fatal outcome and the drug was withdrawn from the market world-wide in 1988.
Chirality and drug withdrawals
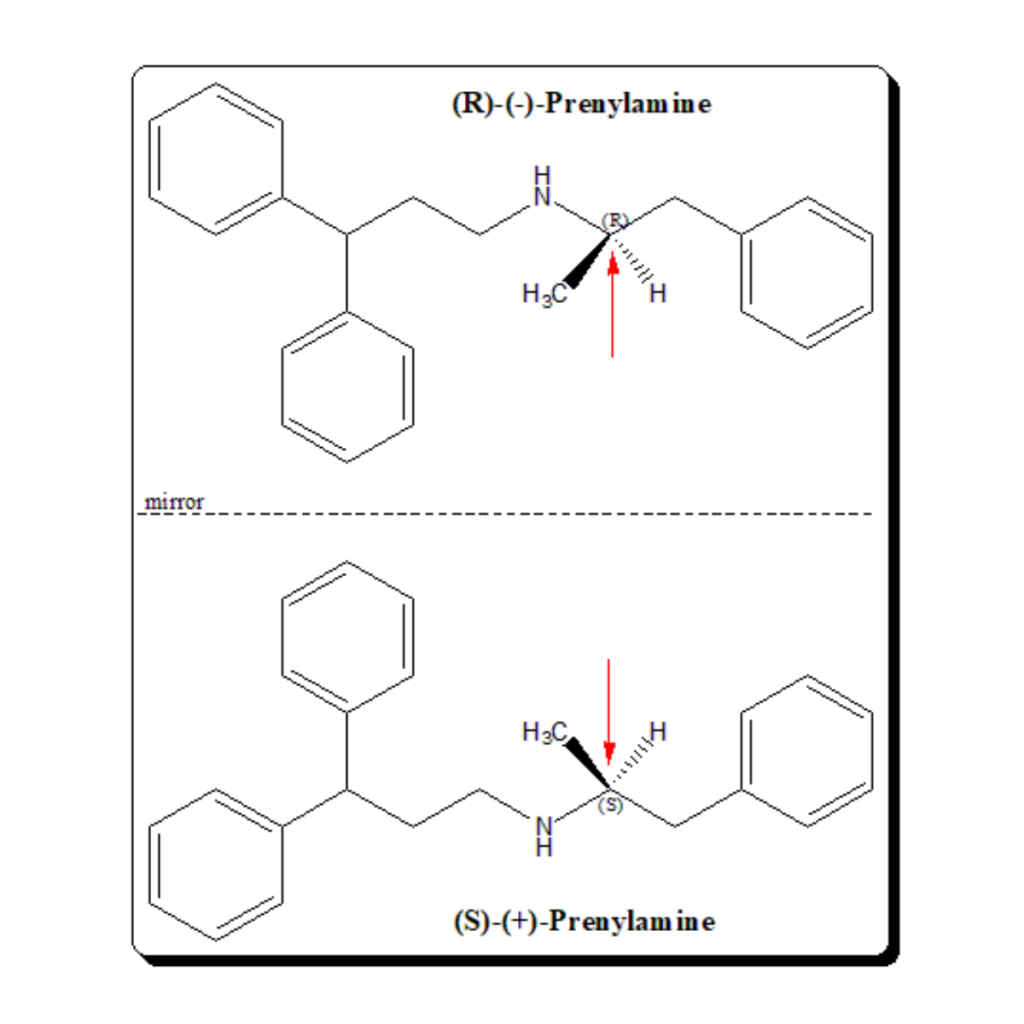
Prenylamine is chemically diphenyl-propyl derivative of phenylalkylamine. The drug is optically active with one stereogenic center (indicated by a red arrows), giving rise to an enantiomeric pair. The product was marketed as a racemic mixture.
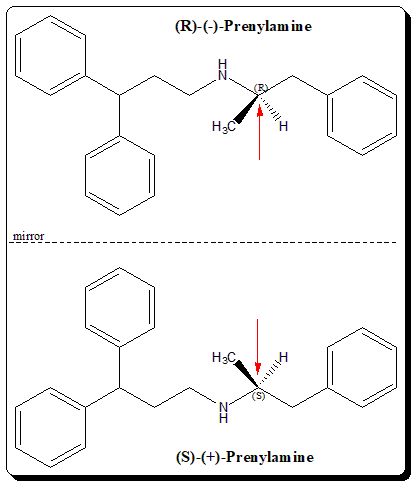
The enantiomeric pair of prenylamine has been shown to exhibit enantiospecific pharmacodynamic profile. In test systems, (R)-(-)-isomer had a negative inotropic effect and shortened the action potential duration. (S)-(+)-enantiomer has positive inotropic action and prolongs action potential duration. Further, the (S)-(+)-prenylamine also found to cause dysrrhythmias (an abnormal rhythm of your heartbeat). Therefore, overall, the data indicate that proarrhythmic effect of (±)prenylamine may have been mediated by (S)-(+)-prenylamine.
Nomenclature
(RS)-N-(1-methyl-2-phenylethyl)-3,3-diphenylpropan-1-amine
Therapeutic category
Antianginal agent – Withdrawn from world market due to enantioselective toxicity
Exercises
Understand the nomenclature and stereo-descriptors employed
References
Rashmi R. Shah and Peter D. Stonier, Withdrawal of prenylamine: perspectives on pharmacological, clinical and regulatory outcomes following the first QT-related casualty. Ther Adv Drug Saf. 2018 Aug; 9(8): 475–493. DOI: 10.1177/2042098618780854
Eichelbaum, Michel F., Testa, Bernard, Somogyi, Andrew (Eds.). Stereochemical aspects of drug action and disposition, Springer, Page 401-32, 2003.