Indacrinone is an orally active, indanone-based loop diuretic patented by American pharmaceutical company Merck and Co as mixture of two enantiomers. In healthy volunteers, the racemic mixture of indacrinone exhibited greater natriuretic potency than furosemide, with a slower onset and longer duration of action. Furthermore, single doses of indacrinone decreased serum uric acid concentrations and increased uric acid clearance, while similar doses of furosemide generally had the opposite effects. Differences in the pharmacologic effects of the resolved enantiomers of indacrinone were initially studied in animals and confirmed in a series of studies we conducted in healthy human volunteer. (Source: https://drugs.ncats.io/drug/AUS78544YM).
Chirality and biological activity
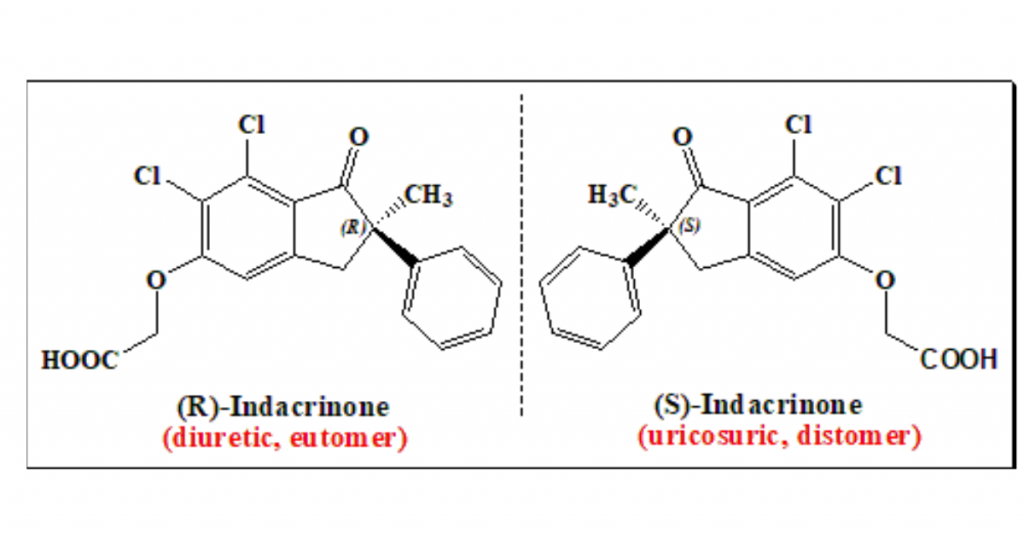
Indacrinone has one stereogenic center and exists as enantiomeric pair. (R)-(+)-enantiomer, the eutomer, is diuretic whereas the mirror-image version (S)-enantiomer antagonizes side effect of the eutomer. To know more about eutomer and distomer nomenclature read @ <https://chiralpedia.com/blog/chiral-twins-identical-but-not-really/>
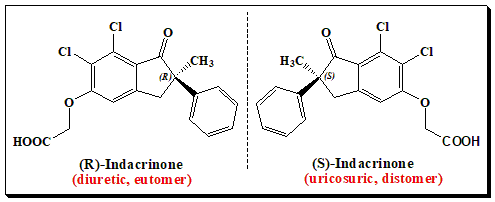
Indacrinone is an interesting example of an enantiomeric pair exhibiting therapeutic advantage. In this case both enantiomers contribute, in different ways, to the overall desired effect. As pointed out earlier the (R)- enantiomer is the pharmacologically active diuretic. Similar to other diuretics, the (R)-isomer possesses the undesirable side-effect of uric acid retention. But the (S)-enantiomer , the distomer, has the property of promoting uric acid secretion (uricosuric effect), and, therefore, antagonizing the undesirable side-effects of the eutomer (uric-acid retention). It provides a good argument for the marketing of a racemic mixture. But studies exemplify that 9:1 mixture of the two enantiomers affords optimal therapeutic value.
Nomenclature
2-[(6,7-dichloro-2-methyl-1-oxo-2-phenyl-3H-inden-5-yl)oxy]acetic acid
Exercise
Understand to assign stereochemical configuration to the chiral center in indacrinone molecule, as per CIP rule (preferably use molecular models)
References
Chiral drugs. Wikipedia, Wikipedia Foundation, 04/07/2022. https://en.wikipedia.org/wiki/Chiral_drugs and references therein
Hassan Y. Aboul-Enein, Irving W. Wainer, The Impact of Stereochemistry on Drug Development and Use, John Wiley & Sons, New York, 1997. https://www.wiley.com/en-
Inxight Drugs, https://drugs.ncats.io/drug/AUS78544YM
Ariëns, Everardus J. “Stereochemistry: A source of problems in medicinal chemistry”. Medicinal Research Reviews. 6 (4): 451–466. 1986.