Dilevalol is the (R,R)-diastereoisomer of labetalol. Labetalol has been in clinical use since 1977 for the treatment of hypertension. Labetalol is an adrenergic antagonist used to treat high blood pressure. It has a role as an antihypertensive agent, a sympatholytic agent, an alpha-adrenergic antagonist and a beta-adrenergic antagonist.
Chirality and drug withdrawals
Labetalol has two stereogenic centers and consequently exists as four stereoisomers. It is a diastereoisomeric mixture of approximately equal amounts of all four possible stereoisomers ((R,S)-labetalol, (S,R)-labetalol, (S,S)-labetalol and (R,R)-labetalol). The (S,S)- and (R,S)- forms are inactive. The third, the (S,R)-isomer, is a powerful α1-blocker. The fourth isomer, the (R,R)-isomer is Dilevalol.
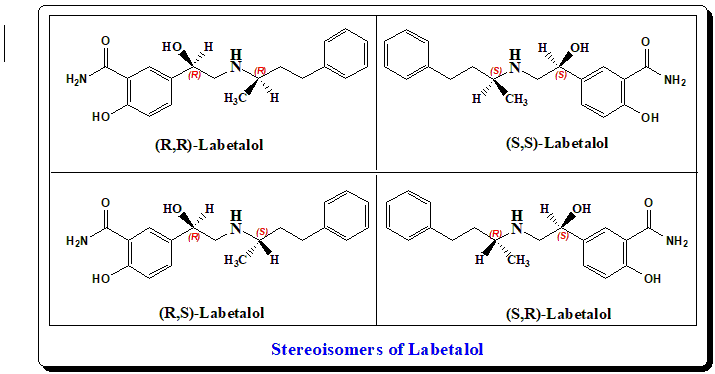
Compared to the racemate, dilevalol has four times β1-adrenorecptor blocking and seven-fold vasodilatory activity due to its agonist activity at the β2-adrernorecpors. Clinically, introduction of dilevalol generated big enthusiasm as it represented a new generation of β-blockers with vasodilating activity. Soon after its introduction in the market in 1989, reports of hepatotoxicity began to appear. Although labetalol is known to manifest hepatotoxicity, the rate of incidence appeared to be lower than that associated with dilevalol. Hence dilevalol was withdrawn from market in 1990.
Nomenclature
(R,R)-labetalol is a 2-hydroxy-5-{1-hydroxy-2-[(4-phenylbutan-2-yl)amino]ethyl} benzamide that has 1R,2R-configuration. 2-Hydroxy-5-((R)-1-hydroxy-2-(((R)-4-phenylbutan-2-yl)amino)ethyl)benzamide
Therapeutic category
Withdrawn from market in 1990 due to stereochemically engineered toxicity
Exercise
Understand the stereo-descriptors employed in the nomenclature
Study the stereoisomeric relationship between all the four stereoisomer of labetalol
References
https://pubchem.ncbi.nlm.nih.gov/compound/Dilevalol
Eichelbaum, Michel F., Testa, Bernard, Somogyi, Andrew (Eds.). Stereochemical aspects of drug action and disposition, Springer, Page 401-32, 2003.