(±)-Terodiline, the antianginal agent, perhaps represents the best authenticated example of a drug that had to be withdrawn from the world market as consequence of proven stereospecific toxicity. Terodiline has a close similarity to prenylamine from a structural and pharmacological view point.
It was firs marketed as an antianginal agent but it exhibited urinary retention as a frequent and worrying side-effect. It was decided to exploit the the side-effect. Therefore the drug was redeveloped and marketed in 1986 for clinical use in urinary incontinence (leaking urine by accident). Over a couple of years reports of serious cardiac toxicity started emerging following the clinical use of terodiline and some of them had a critical outcome. Because of these observations, in 1991, terodiline was withdrawn from the market world-wide
Chirality and drug withdrawals
Terodiline has one stereogenic center and exists as mirror-image twins. Terodiline being structurally related to prenylamine demonstrates enantioselective pharmacological effects. The calcium agonist activity remains predominantly in the (S)-(-)-terodiline, whereas the anticholinergic actions is found in the (R)-(+)-enantiomer. Both the pharmacodynamic actions possibly contribute to the overall therapeutic effect to a variable extent.
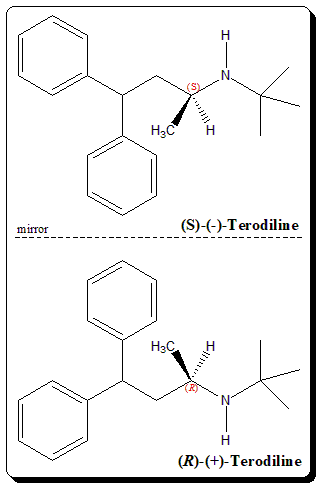
Enantioselective studies has shown that the (R )-(+)-terodiline is responsible for the prolongation of the QT interval associated with the racemic terodiline. Hence, the (R)-enantiomer is found to be the culprit for the serious ventricular tachyarrhythmias observed with the clinical use of this drug.
Nomenclature
(RS)-N-tert-butyl-4,4-diphenylbutan-2-amine (RS)-N-tert-Butyl-1-methyl-3,3-diphenylpropylamine
Therapeutic category
Antianginal and treat urinary incontinence – Withdrawn from world market due to stereospecific toxicity
Exercise
Learn to draw stereochemical structure, understand stereo-descriptors employed in the nomenclature system
References
https://pubchem.ncbi.nlm.nih.gov/compound/Terodiline
Eichelbaum, Michel F., Testa, Bernard, Somogyi, Andrew (Eds.). Stereochemical aspects of drug action and disposition, Springer, Page 401-32, 2003.

Thank you sir for sharing the information on importance of stereochemistry by giving very specific chiral molecule in different aspects. Thanks to “chiralpedia” for its contribution to the field of chiral science. Thank you sir.