In nature, most chiral molecules only appear in one specific enantiomeric form. [Read more @ the blog – “The handed world“, https://chiralpedia.com/blog/the-handed-world/]. Moreover, the handedness of the enantiomer can have a powerful effect on how that molecule behaves. The chiral difference between two molecules often plays a huge role in pharmacology and physiology, even though the two seem nearly identical but are not really in a bioenvironment, which is chiral. [Read more @ the blog -“Chiral twins – Identical? … But not really!”, https://chiralpedia.com/blog/chiral-twins-identical-but-not-really/]. For example, one enantiomer of the chiral molecule limonene smells of lemon, while the other version smells of orange. An extreme consequence of the difference in the pharmacological property of a chiral twin is exhibited by the drug thalidomide. One of the chiral twins of thalidomide cure morning sickness while the other lead to deformed children being born. Because of this differing biological profile of the enantiomeric pair, separating chiral substances has researchers’ attention.

Separating mirrored molecules has been difficult and a challenge since their chemical properties are essentially identical. There is a need to construct a chiral environment to resolve them. Lot of progress has been made in the field of chiral resolution of chiral twins. Wide range of techniques starting from the classical chiral chromatography to novel approaches like chiral molecular sieves, chiral membranes, chiral nanosheets, magnets has been developed for separating mirrored molecules.
Chiral chromatography
” Chiral chromatography has advanced to turn into the most preferred technique for the determination of enantiomeric purity as well as separation of pure enantiomers both on analytical and preparative scale. Chiral chromatographic assay is the first step in any study pertaining to enantioselective synthesis or separation. This includes the use of techniques viz. gas chromatography (GC), high performance liquid chromatography (HPLC), chiral supercritical fluid chromatography (SFC), capillary electrophoresis (CE) and thin-layer chromatography (TLC). The result of a literature survey done identifies HPLC-based chiral assays as the most dominating technology in use”.
Read more @ Wikipedia article link: “Chiral analysis” Wikipedia, Wikimedia Foundation, 02/15/2022, https://en.wikipedia.org/wiki/Chiral_analysis
Molecular sieves
“The synthesis of a chiral molecular sieve has been of great relevance significance since this type of material could open new opportunities for asymmetric catalysis and chiral separations. The robust nature of these porous solids could show commercial advantages in chiral processes, as these materials are easily regenerated and thus used for many cycles of performance. This characteristic alone would likely provide significant cost efficiency over other chiral agents that can only be used for a limited number of cycles as they tend to decay in some way. Enantiomerically enriched, polycrystalline molecular sieve has been prepared and shown to function for chiral adsorption and catalysis. As far as applications are concerned, chiral separation and catalytic processes are evident directions for investigation”.
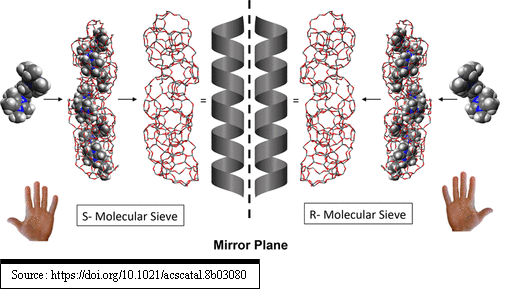
Continue reading in the following link:
Mark E. Davis, A Thirty-Year Journey to the Creation of the First Enantiomerically Enriched Molecular Sieve. https://pubs.acs.org/doi/10.1021/acscatal.8b03080
Chiral membranes
“Membrane-based enantioseparation technique has the potential for large-scale production of enantiopure compounds. Of late adsorption-type enantioselective membranes and membrane-assisted separation systems with non-enantioselective solid membranes have drawn much attention. The principles and recent developments of both enantioselective liquid and solid membranes and membrane-assisted processes for chiral resolution will be of interest to a wide range of readers from diversified fields viz. analytical, organic and medicinal chemistry, to pharmaceutics and materials, to process engineering for manufacturing pharmaceuticals, agrochemicals, fragrances and foods, and so on.
Read more in the following link: Rui Xie et al., Membranes and membrane processes for chiral resolution, Chem. Soc. Rev., 2008, 37, 1243–1263 | DOI: 10.1039/b713350b. https://pubs.rsc.org/en/content/articlelanding/2008/cs/b713350b
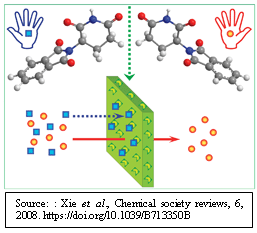
” Recently, Prof. LIU Bo and group at the University of Science and Technology of China (USTC) have developed a chiral separation membrane able to hold left-handed chiral molecules and release right-handed version using two-dimensional layered materials. The chiral membrane, exhibits separation efficiency up to 89% towards limonene racemate, is expected to be apply the technology for industrial production. Currently, the researchers are able to make chiral membranes in the laboratory scale. Now, the team is augmenting the membrane size to meet the requirement of pharmaceutical industry for separating chiral drugs”.
Continue reading in the following link: Wang, Y., Wu, N., Wang, Y. et al., Graphite phase carbon nitride based membrane for selective permeation. Nat Commun 10, 2500 (2019). https://doi.org/10.1038/s41467-019-10381-z
Magnets
“Another interesting discovery is the use of magnets to sort enantiomers, by the scientists at Hebrew University of Jerusalem in Israel, a cheap and simple method. Their research findings have been published in journal Science. In this technique the magnet is placed in a solution containing both enantiomers – one enantiomer crystallizes on the north pole of the magnet, and the other enantiomer crystallizes on the South Pole. This happens due to the electrons. Electrons have a property called ‘spin’, which can be positive or negative.
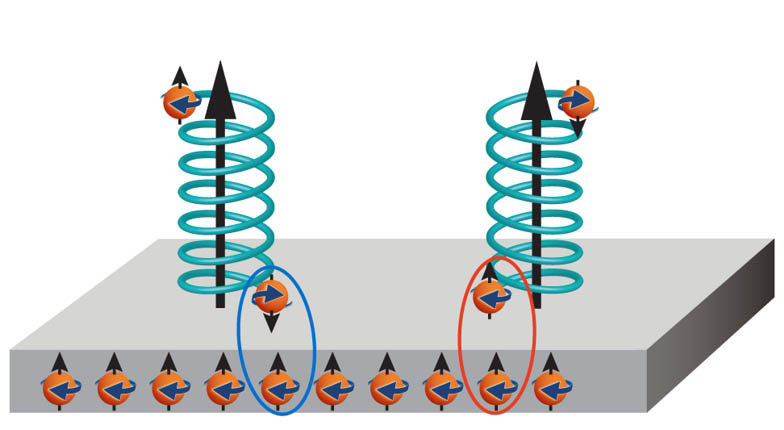
Ref: K. Banerjee-Ghosh et al., Science (2018), doi: https://doi.org/10.1126/science.aar4265
When a molecule interacts with a surface, it becomes ‘spin-polarized’, meaning that electrons with a particular spin accumulate at one end of the molecule. The direction in which polarization occurs depends on the chirality of the molecule. At the pole of a magnet, all the spins are aligned in the same direction. This means that when a chiral molecule approaches the magnet and becomes spin-polarised, the end of the molecule that is pointing to the surface of the magnet can have either the same spin or the opposite spin to the electrons in the magnet. If the spins are opposite the molecule is attracted to the surface. If the spins are the same, the molecule is repelled. In this way, each enantiomer will collect at a different pole of the magnet. This open up new avenue for a different approach to chiral separations”.
Read more in the following link:
Koyel Banerjee Ghosh, et al., Separation of enantiomers by their enantiospecific interaction with achiral magnetic substrates. https://www.science.org/doi/10.1126/science.aar4265
Using magnets to separate chiral molecules, https://www.rsc.org/news-events/journals-highlights/2019/may/magnets-separate-chiral-molecules/

Thanks for sharing this informations..