In the 1940s and early 1950s, pregnant women throughout the world who experienced morning sickness and other anxieties associated with the first term of pregnancy were given barbiturate sedatives for relief. The issue with these barbiturates, like most sedatives, was the fact that they were highly toxic in large doses. In 1957, Chemie Grunenthal of Germany launched thalidomide as a safer alternative to barbiturate sedatives. Thalidomide, as a new sedative, was a very “attractive” drug in that it was not at all toxic even when overdosed in large quantities, as compared to the other lethal sedatives in use.
Teratogenicity: drug induced toxicity
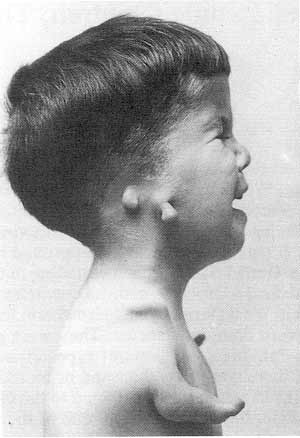
Thalidomide was sold over the counter and by prescription by many firms in many countries under license from the parent company, being very effective in lessening morning sickness and very effective as a sleeping pill. Eventually, children in 46 countries were affected. Thalidomide was not a lifesaving drug, but only one of many tranquilizers that had come onto the market in the decade after World War II. It was promoted by its manufacturer as being nontoxic, with no side effects, and completely safe for pregnant women. Not one of those statements was true. In addition to the teratogenic effect on the foetus, in adults it caused peripheral neuritis, a painful numbing of the hands and feet that is often irreversible, as a side effect. There were scientific tests that, had they been conducted, might have shown thalidomide to be unsafe. The drug companies involved, however, did not perform those tests.The years following the initiation of thalidomide use throughout Europe were blemished with countless reports of severely deformed children being born. Mothers were giving birth to babies with a clinical condition referred to as “phocomelia” (deformed hands and limbs).
Thalidomide: a look in the mirror
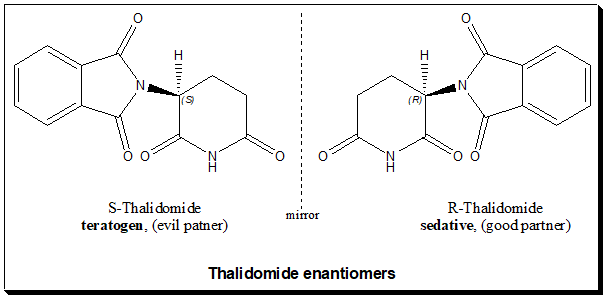
Thalidomide is a glutamic acid derivative. The molecule is a two ringed structure with a stereogenic carbon in the glutarimide ring. Hencel exists as an equimolar mixture of S(-)- and R(+)-enantiomers. It was marketed as racemic mixture. The stereogenic carbon in thalidomide is highly labile in protonated aqueous media and undergoes configurational inversion otherwise referred to as chiral inversion. Hence, the individual enantiomers of thalidomide are both inverted rapidly to the racemic mixture.
Thalidomide: chiral twins
There has been research carried for the separation and quantification of both enantiomers of thalidomide for enantiospecific pharmacokinetic, pharmacodynamic and toxicologic investigations. Separation techniques employed to resolve racemic mixtures include chiral High Performance Liquid Chromatography (HPLC) and chiral capillary electrophoreses (CE).
The primary study addressing this problem, using oral administration to rabbits and mice, showed that both the enantiomers were equally teratogenic and had the same sedative effect. A subsequent study, with the pure enantiomers of thalidomide, established that the (R)-(+)- enantiomer has the desired effect whereas the (S)-(-)-enantiomer harbors the undesired teratogenic effect in both rabbits and mice. This study gave rise to the belief that the thalidomide disaster might have been avoided if only the pure (R)-enantiomer has been used. More recent research has confirmed that after in vivo administration of either of the two enantiomers to the humans, there is a rapid interconversion between the two forms. The two enantiomers are interconverted far too quickly in the liver, in vivo racemization takes about 8 hours. So there is little point in making the drug in single isomer form, as it would not remove the risk of the (S)-enantiomer. Hence the proposal that the thalidomide tragedy could have been avoided if the (R)-enantiomer has been used is misleading.
Thalidomide – the return
The word ‘thalidomide’ became synonymous with tragedy throughout the world. However, thalidomide did not disappear from the “arsenal of medicines” of doctors. Today, almost 40 years later, manufacturers are conducting clinical trials to see if thalidomide can treat inflammation common to a host of diseases, and combat weight loss and aphthous ulcers in AIDS patients. Other trials are studying thalidomide’s effect on the eye disease macular degeneration; on breast, prostate and brain cancer; and on Kaposi’s sarcoma (a form of cancer common in AIDS patients). If thalidomide is shown to be safe and effective for even one such use, it would represent a remarkable comeback for a drug that once was universally condemned. Despite its history as a human teratogen, thalidomide is emerging as a treatment for cancer and inflammatory diseases.
As pointed out earlier, the stereogenic center in thalidomide is very labile and because of this the molecule has a tendency to racemize spontaneously through chiral inversion. So research efforts are on to synthesize derivative of thalidomide with a hope to separate the good from the evil partner. Celegene, a pharmaceutical company involved in the development of thalidomide drug therapy, is currently working on developing a new category of drugs called IMiDs (Immunomodulatory Drugs, example – lenalidomide) and SelCIDs (Selective Cytokine Inhibitory Drugs). These new drugs are thalidomide derivatives, which are advertised as being more potent and less dangerous than thalidomide itself. Studies in animal models have shown enhanced anti-angiogenic and TNF-alpha inhibiting properties. These studies have also demonstrated that the IMiDs do not cause birth defects. Celegene is hopeful in furthering its studies to human test subjects in the near future.
Food & Drug Administration approved thalidomide in the U.S. for the use of treatment with patients afflicted with leprosy. However, this treatment comes with conditions and guidelines for both, the physician and the patient. Among other rules and regulations, Celegene Corporation, the company now manufacturing thalidomide has developed a registry called STEPS, System for Thalidomide Education and Prescribing Safety programme. Only physicians registered can administer and prescribe thalidomide. Both male and female patients have to undergo detailed counseling, education, surveys, and frequent follow up studies in order to be prescribed and go on with thalidomide treatment.
Thalidomide tragedy: lessons
Thalidomide history, the tragedy and its misuse in the past, serves as a lesson in drug development that includes:
- Draws attention to the prominence of considering data in full and not leaping into conclusions, however tempting these might be.
- Difficult to evaluate and extrapolate the observed stereoselective toxicity data in animals to the disaster in humans
- Demonstrates that chemicals like thalidomide are not inherently evil but the morality resides only in the hands of those who use them.
- Emphasize the need to understand the molecular pharmacology of a compound’s activity, including associated toxicities from a mirror-image perspective if the molecule is handed.
Further reading
1. A curse on my baby. Sunday Times, Focus, July 20, 1997.
2. Suffer the children: the story of thalidomide, Sunday times (UK), 1979.
3. Eriksson, T., Björkman, S., Roth, B., Fyge, A., Höglund, P., Stereospecific determination, chiral inversion in vitro and pharmacokinetics in humans of the enantiomers of thalidomide. Chirality,7(1):44-52, 1995. DOI: 10.1002/chir.530070109
4. What is Thalidomide? TVAC: Thalidomide Victims Association of Canada, September 01, 1999. Available: http://www.thalidomide.ca
5. Thalidomide: A Medical Dictionary, Bibliography, and Annotated Research Guide to Internet References / James N. Parker and Philip M. Parker, editors, ICON Health Publications, ICON Group International, Inc. USA, 2004.
6. Silas W. Smith, Chiral Toxicology: It’s the Same Thing…Only Different. Toxicological Sciences, Volume 110, Issue 1, , Pages 4–30, 2009. https://doi.org/10.1093/toxsci/kfp097
7. Tokunaga, E., Yamamoto, T., Ito, E. et al. Understanding the Thalidomide Chirality in Biological Processes by the Self-disproportionation of Enantiomers. Sci Rep 8, 17131 (2018). https://doi.org/10.1038/s41598-018-35457-6
Nice and useful article… Much appreciated 👏
Thank you.