Chemical Compounds that come as mirror-image pairs are referred to by chemists as chiral molecules or chiral twins. Drugs that exhibit handedness are referred to as chiral drugs. The most commonly encountered stereogenic unit that confers handedness to drug molecules is stereogenic center or chiral center. Many medicinal agents important life are combinations of chiral twins in which one partner may cure a disease, quell headache or smell good, while its mirror image may be poisonous, smell nauseating or simply the inert.

The tragedy of thalidomide illustrates the potential for extreme consequences resulting from the administration of a racemate drug that exhibits multiple effects attributable to individual enantiomers. Doctors prescribed it as a sedative and sleeping medicine for pregnant women. Thalidomide molecule carries one chiral carbon and the drug was made and marketed as a racemic mixture of the (+)(R)-thalidomide and (-)(S)-thalidomide.
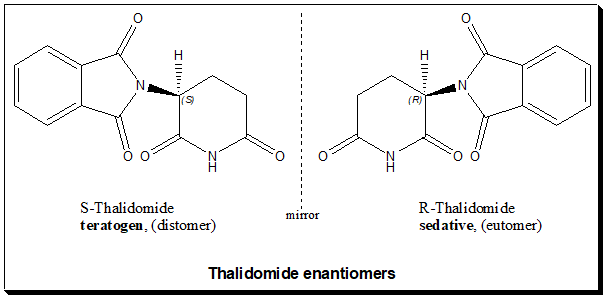
In the 1960, hundreds of European babies whose mothers had taken the sedative thalidomide, marketed as a mixture of the left- and right-handed versions, were born with deformed babies, referred to as thalidomide babies. The (R)-enantiomer has the desired sedative effects, whereas the (S)-isomer is teratogenic. Teratogen is a substance affecting the development of the foetus and causing structural or functional disability. As a result of this thalidomide disaster, many countries began to tighten drug approval process.
Understanding biological action
The initiation of biological effects, desired (pharmacological) and undesirable (toxicological) is based on a complex sequence of chemical and physicochemical processes. After the administration, a drug molecule has to traverse through a number of bioloical phases, before reaching the target tissue. For an understanding of drug action, it is efficient to distinguish three main phases (Refer Figure)
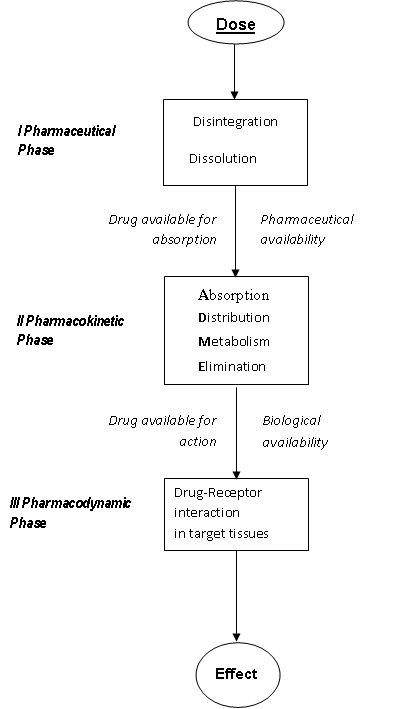
The pharmaceutical phase comprises the processes that are determinant for the efficacy of the dosage form. The fraction of the dose available for absorption is indicated as the “Pharmaceutical availability”. In general, for the absorption, which implies passage through biological membranes, the lipid/water solubility and therewith the partition coefficient is determinant. The pharmacokinetic (what body does to the drug) phase is composed of processes involved in absorption, distribution, excretion and metabolic conversion of the agent after absorption. The fraction of the dose that reaches the general circulation is indicated as the “biological (systemic) availability”. Enzymes chiefly mediate the ADME processes. The pharmacodynamic (what body does to the drug) phase constitutes the processes involved in the interaction between the bioactive compounds and their molecular sites of action, receptors, enzymes, etc. Pharmacon-receptor interaction results in the induction of a stimulus that initiates a sequence of biochemical and biophysical events, which finally lead to the effect, observed. In most cases this interaction is influenced by the three-dimensional characteristics of both the Pharmacon and the receptor.
Bio-environment and chiral twins
The Behaviour of the chiral twins depends mainly on the nature of the environment (achiral/chiral) in which they are present. An achiral environment does not differentiate the chiral twins whereas a chiral environment does distinguish the left-handed from the right-handed version. This concept can be better appreciated by the following analogy.
Feet are chiral, i.e. exhibit handedness. But it does not matter which socks goes onto which foot, because socks is achiral; however, shoes are chiral, so each foot will interact differently with a particular shoe. Here feet are compared to drug enantiomers and shoes to chiral biological matrices.
Human body, a classic bio-environment, is inherently handed as it is filled with chiral discriminators like amino acids, enzymes, carbohydrates, lipids, nucleic acids etc. Hence when a racemic therapeutic gets exposed to biological system the component enantiomers will be acted upon stereoselectively. For drugs, chiral discrimination can take place either in the pharmacokinetic or pharmacodynamic phase. In both phases, interaction with a chiral macromolecule results in the formation of two energetically distinct, diastereoisomeric complexes, which have different physiochemical properties.
For example, the interaction between the Body (R) and each of the chiral drugs twins (R and S) can be depicted chemically as below.
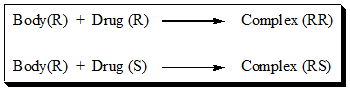
The complex (RR) and (RS) are related to each other as diastereomers. Such differences make, drug enantiomers biologically distinguishable and possibly give rise to pharmacological and toxicological effects that seems to be the root cause of the growing concerns about chiral drugs.
There has been lot of criticisms about the practice of conducting pharmacokinetic and pharmacodynamic studies on racemic therapeutics and ignoring the separate contributions of the individual enantiomers. Further the renewed awareness of the three-dimensional effects of drug action fuelled by the exponential explosion of chiral technology emerged the new area “Stereopharmacology”. A more specific term is ‘Chiral pharmacology” a phrase popularized by Professor John Caldwell.
Chiral pharmacology
This field has grown itself into a specialized discipline concerned with the three-dimensional aspects of drug action and disposition. This approach essentially views each version of the chiral twins as separate chemical species. Racemic drugs are not drug combinations in the accepted sense of two or more co-formulated therapeutic agents, but combinations of isomeric substances whose pharmacological activity may reside predominantly in one specific enantiomeric form. The majority of these racemic mixtures of synthetic chiral drugs the use of these can be thought of as polypharmacy, with the proportions of the various enantiomeric forms present being dictated by chemical rather than pharmacological or therapeutic criteria.
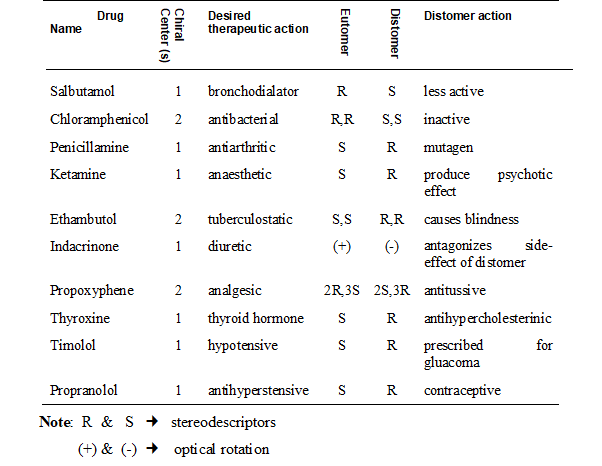
To express the pharmacological activities of each of the chiral twins two technical terms have been coined eutomer and distomer. The member of the chiral twin that has greater physiological activity is referred to as the eutomer and the other one with lesser activity is referred tom as distomer. The eutomer/distomer ratio is called the eudisimic ratio and reflects the degree of enantioselectivity of the biological activity.
In case of stereoselectivity in action only one of the components in the racemic mixture is truly active. The other isomer, the distomer, should be regarded as impurity or isomeric ballast not contributing to the effects aimed at. As pointed out earlier it is well appreciated that the pharmacologically inactive isomer (distomer) may contribute to the toxicity or adverse effects of the drugs. There is a wide spectrum of possibilities of distomer actions, many of which are confirmed experimentally. The table presents selected examples to demonstrate the wildly differing biological effects observed between enantiomeric pairs.
As a consequence of the wildly differing pharmacological effects exhibited by chiral twins efforts are being made by scientist to develop technologies to remove the evil twin. By choosing only the useful chiral twin during drug manufacturing could help produce pure drugs that do not cause adverse effects.
References
1. Crossely, R. Tetrahedron, 1992, 48, 8155-8178.
2. Gross, M. Ann. Rep. Med. Chem., 1990, 25.323-331.
3. Scott, A, K. Drug lnd. J.,1990, 24,121-123.
4. Wainer, l. W., & Drayer, D. E., Eds. Drug stereochemistry and Pharmacology: Marcel-Dekker, New York,1988.
5. Ariëns, E. J. Med. Res. Rev.1986. 6, 451-466.
6. John Caldwell. Chiral Pharmacology and the regulation of new drugs, Chemistry and Industry, 6 March, 1995, 176-179.
7. Fassihi, A. R. lnt. J. Pharm.,1993, 92,1-14.
8. Wainer, I. W. Am. J. Hosp. Pharm.,1992, 49, Suppl.1, S4-S8.
9. Decamp, W. H. J. Pharm. Biomed. Anal.,1993.11, 1167-1172.
10. Purer, safer drugs by removing the evil twin. https://www.asiaresearchnews.com/content/purer-safer-drugs-removing-evil-twin
11. Chiral drugs. Wikipedia, Wikipedia Foundation, 27/01/2022. https://en.wikipedia.org/wiki/Chiral_drugs


An Interesting and informative blog on chiral pharmaceutical drugs which are Identical? … But not really!. The contents of the blog is interesting and provided useful information. This, Blog is really helpful for us. Thank You sir.
Thanks for this nice post. Gives a gentle induction to noobs into the facinating chiral chemistry. It proves the point that a well written blog is worth a thousand textbook pages. Looking forward for more.