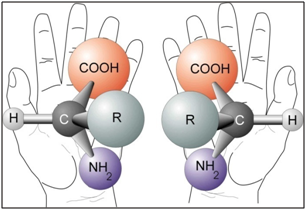
We live in a chiral world!
Many important molecules required for life exist in two versions that are mirror images of each other. They are related like our left and right hands, but they are not the same. This property is called ‘chirality’, from the Greek word cheir for hand, or handedness in a general sense and the two forms are called ‘enantiomers’, from the Greek word for opposite. This phenomenon is called chirality. Other synonyms commonly used are handedness, or enantiomerism.
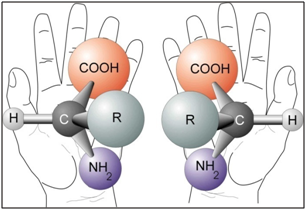
Complex enzymatic systems and simpler building blocks of life: proteins, amino acids, sugars, etc. are chiral. One may well think that both forms of chiral molecules ought to be equally common in nature the reactions should be symmetrical. But when we study the molecules of the cells in close-up, it is evident that nature mainly uses one of the two enantiomers.
Nature has been making skillfully precise single-isomer chirality since the very early stages of evolutionary biochemistry. In human body, proteins and DNA possess a unique 3-dimensional shape, and it is because of this 3D shape that the biochemical processes within our bodies work as they do. It is chirality that provides the unique shape for proteins and DNA, and without chirality, the biochemical processes in our bodies would not do their job. In our body, all 20 naturally-occurring amino acids – forming the backbone of all proteins, enzymes and hormones in living organisms – as well as both DNA and RNA nucleic acids – are found with the same chirality, L-amino acids. All the sugars that go into the making of nucleic acids are D-sugars.
As nucleotide molecules come together to form the structure of DNA, they develop a twist that forms the double helix structure of DNA. DNA develops a twist in the chain because each component contains chirality or handedness. It is this handedness that gives DNA the spiral shaped helical structure. If one molecule in the DNA structure had the wrong chirality, DNA would not exist in the double helix form, and DNA would not function properly. The entire replication process would be derailed like a train on bad railroad tracks.

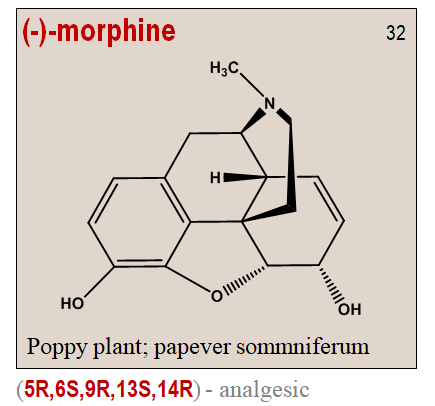
It is interesting to note that unichirality is hall mark of nature. To cite an example from plant kingdom, the poppy plant Papaver somniferum only synthesizes the pain reliving (-)-morphine. Morphine is a highly demanding chemical structure with 5 chiral centers and hence can exist in 32 stereoisomeric forms. But the levo-morphine has the desired narcotic analgesic activity with the configuration (5R,6S,9R,13S,14R).
Another example is the antimalarial agent Quinine, which has 4 chiral centers and can exist in 16 stereoisomeric forms. But only the (-)-form with configuration (3R,4S,8S,9R) exhibits the biological activity.
Chirality in daily lives
Limonene and Caravone famously demonstrate the significance of stereochemistry even in everyday life. Limonene, for example, is chiral, but the two enantiomers can be difficult to distinguish at first glance. The olfactory receptors in our nose are more sensitive and the nose knows!!!. One form certainly smells of lemons but the other of oranges. This is a classical example to demonstrate how differently the two enantiomers can affect our cells.
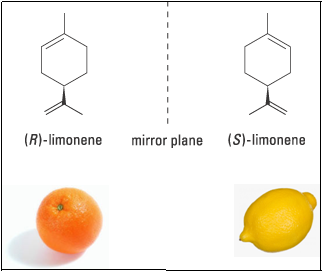
When configuration of a molecule is change, say from (R)- to (S)-, essentially change in the shape of the molecule is happening. In one shape the molecule is triggering olfactory receptor in the nasal tract responsible for the orange smell while in the other shape it is triggering the receptor responsible for the lemon smell.
Thus the enzymes in our cells are chiral, as are other receptors that play an important part in cell machinery. This means that they prefer to bind to one of the enantiomers. In other words, the receptors are extremely selective; only one of the enantiomers fits the receptor’s site like a key that fits a lock. Since the two enantiomers of a chiral molecule often have totally different effects on cells, it is important to be able to produce each of the two forms pure.

The importance of stereochemistry in biological systems extends to more than just drugs: our bodies, for example, can only create and digest carbohydrates and amino acids of a certain stereochemistry. Thus, all of the proteins that make up our hair, skin, organs, brain, and tissues, are composed of a single stereoisomer of amino acids. Additionally, our bodies can make and digest starch (found in potatoes and bread) but not cellulose (found in wood and plant fibers), even though both are just polymers of glucose of different stereochemistry. These are just a few of numerous examples of the important role stereochemistry plays in our everyday lives.
Chirality in Pharmaceuticals
Most of the medicinal agents are mirror-image twins and since a drug must match the molecules it should bind to itself in the cells, it is often only one of the enantiomers that is of interest. Understanding chirality is extremely important in the preparation of therapeutic drugs. For example, one enantiomer of penicillamine is a potent anti-arthritic agent whereas the other enantiomer is highly toxic. Perhaps the most startling example of the difference in activity between enantiomers is Thalidomide. This drug was seen as a panacea for the treatment of morning sickness in pregnant women, and indeed one enantiomer reliably has this effect. The other enantiomer, unfortunately, has been associated with the well-characterized birth defects that arose from use of Thalidomide called phocomelia (a rare birth defect that can affect the upper and/or lower limbs).
Clearly mechanisms for both health and disease therefore have a chiral basis at the molecular level. Effectively designed drugs would therefore be expected to also be developed with the appropriate exact chirality and regulatory authorities are insisting on the development of enantiopure medicines. In the past a mixture of the two enantiomers was routinely employed since a mixture is much easier to produce than a single enantiomer. Unfortunately, in certain cases the other enantiomer may be harmful, as was the case in the thalidomide disaster in the 1960s
As we see chirality is important not only in medicinal chemistry, but also in perfume chemistry, agrochemicals and properties of materials. It has thus become big business. Consequently, there has been intensive research into developing methods for making one or other of the enantiomers – in other words, in being able to control chirality. It is interesting to note that Benjamin List and David MacMillan were awarded the Nobel Prize in Chemistry 2021 for their development of a precise new tool for molecular construction: organocatalysis, that can make specific mirror images of molecules.
References
1. Noemie Globus and Roger D. Blandford, The chiral puzzle of life. https://iopscience.iop.org/article/10.3847/2041-8213/ab8dc6.
2. Stephen Mason, The origin of chirality in nature. https://www.sciencedirect.com/science/article/abs/pii/016561478690235X.
3. Donna G. Balckmond, The origin of biological homochirality. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2857173/#:~:text=The%20property%20of%20chirality%E2%80%94nonsuperimposable,t%20Hoff%20and%20Le%20Bel
4. Al Arsh Basheer, Chemical chiral pollution: Impact on the society and science and need of the regulations in the 21st century. https://onlinelibrary.wiley.com/doi/10.1002/chir.22808
5. Chiral drugs. Wikipedia, Wikipedia Foundation, 06/01/2022. https://en.wikipedia.org/wiki/Chiral_drugs
6. Press release: The Nobel Prize in Chemistry 2021. https://www.nobelprize.org/prizes/chemistry/2021/press-release/

Nice article.
Nice article sir, The title The handed world… looks fresh. I like the statement where you mentioned “unichirality is hall mark of nature” is interesting. We live in a chiral world! is nicely connected through Chirality in daily lives topic. Thank you sharing the information sir.
Regards
K.Selvakumar