Lead
Chirality, the property of molecules having non-superimposable mirror images, plays a crucial role in pharmaceuticals. These molecules, referred to as enantiomers, chiral twins, mirror-image, or handed molecules, are crucial in drug design and effectiveness. A significant proportion of chiral drugs on the market are available as racemic mixture. The handedness of these molecules can lead to significantly different biological activities and therapeutic outcomes. This blog delves into the importance of chirality in drug design and effectiveness, highlights key case studies of chiral drugs, and explores the challenges and advancements in chiral drug synthesis. Understanding the role of chirality in medicine enhances our knowledge of drug action and drives innovation in the pharmaceutical industry.
How Chirality Affects Drug Action and Disposition
Understanding Events of Biological action
After oral administration of a racemic chiral drug, it travels through a sequence of events: the pharmaceutical phase, pharmacokinetic phase (ADME processes), and pharmacodynamic phase (drug-receptor interaction). All these biological processes are mediated by enzymes, which are mostly chiral, leading to chiral discrimination in any of the phases – pharmaceutical, pharmacokinetic, or pharmacodynamic.
The different biological activities of enantiomers arise from their interactions with chiral environments within the body, such as enzymes, receptors, and proteins. One enantiomer may bind effectively to a target receptor and elicit a desired therapeutic effect, while the other enantiomer may be inactive or even harmful. This aspect will be discussed further in the section on chiral drugs and biological activity.
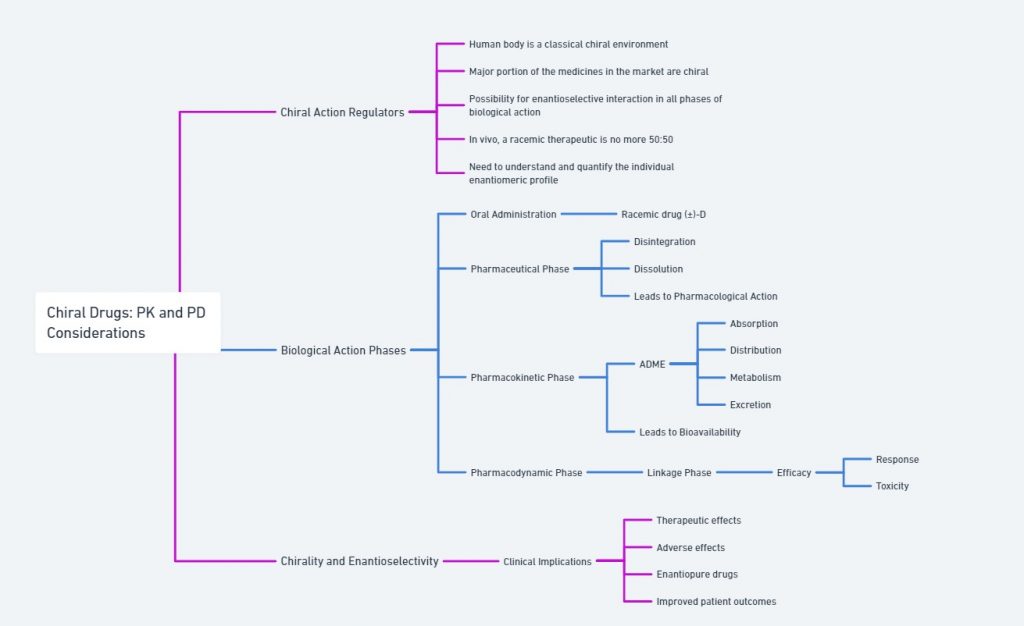
Bio-environment and handed molecules
The human body, a classic bio-environment, is inherently handed, filled with chiral discriminators like amino acids, enzymes, carbohydrates, lipids, and nucleic acids. When a racemic therapeutic enters the biological system, the component enantiomers are acted upon stereoselectively by the various biological actions (ADME process). Consequently, the enantiomeric pair may exhibit wildly differing pharmacokinetic and pharmacodynamic properties. Therefore, in a bio-environment, it is crucial to track the component enantiomers of a chiral drug separately.
Chiral drugs and biological activity
Many medicinal agents are combinations of mirror-image twins. Despite their close resemblance, the differences in their biological properties can be profound. The component enantiomers of a racemic chiral drug may differ significantly in their pharmacokinetic and pharmacodynamic profiles.
Two technical terms, eutomer and distomer, express the pharmacological activities of each chiral twin of a racemic drug. The eutomer is the enantiomer with greater physiological activity, while the distomer has lesser activity. In stereoselective action, only the eutomer is truly active, while the distomer is considered an impurity or isomeric ballast, not contributing to the intended effects. The pharmacologically inactive isomer (distomer) may contribute to the drug’s toxic or adverse effects. The distomer actions can range from equipotent to inactive, undesirable/toxic, complimentary, or having different therapeutic values, many of which are confirmed experimentally.
Examples of Enantiomer-Specific Drug Effects (select)
The image below depicts the chirality concept, technical term employed to express the stereoselectivity in action only one of the components in the racemic chiral drug and the benefits of enantiopure or unichiral drug.
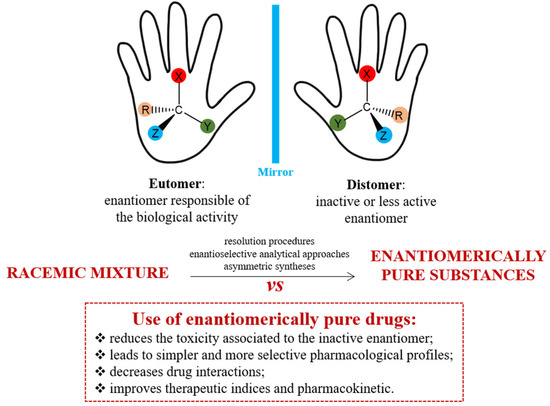
Advantages in the use of enantiopure drugs
Chirality is a fundamental property of ‘handedness’ and is crucial in the development of many pharmaceuticals. Despite this, a significant number of pharmaceuticals continue to be marketed and administered as racemates. Chirality is omnipresent, both in the external environment and within our bodies; receptors and enzymes, which are inherently chiral, interact specifically with chiral drugs. Therefore, controlling enantiomeric purity and isolating enantiomers from chiral drugs is essential for analytical, clinical, and regulatory purposes, enhancing the drug safety profile. Classic examples of spontaneous enantiomerization and severe toxicity associated with chirality include ibuprofen and thalidomide, with numerous other cases documented in the literature..
Case Studies
Selected case studies of chiral drugs representing wildly differing biological properties of the enantiomers are presented to highlight the impact of of chirality on the pharmacological profile of chiral twins.
Active vs. Inactive
Ibuprofen, a widely used non-steroidal anti-inflammatory drug (NSAID), is a classic example. The S-enantiomer of ibuprofen is responsible for its anti-inflammatory and analgesic effects, while the R-enantiomer is largely inactive. Despite this, ibuprofen is often administered as a racemic mixture containing both enantiomers.
Beneficial vs. Harmful
The tragedy of thalidomide illustrates the potential for extreme consequences resulting from the administration of a racemate drug that exhibits multiple effects attributable to individual enantiomers.
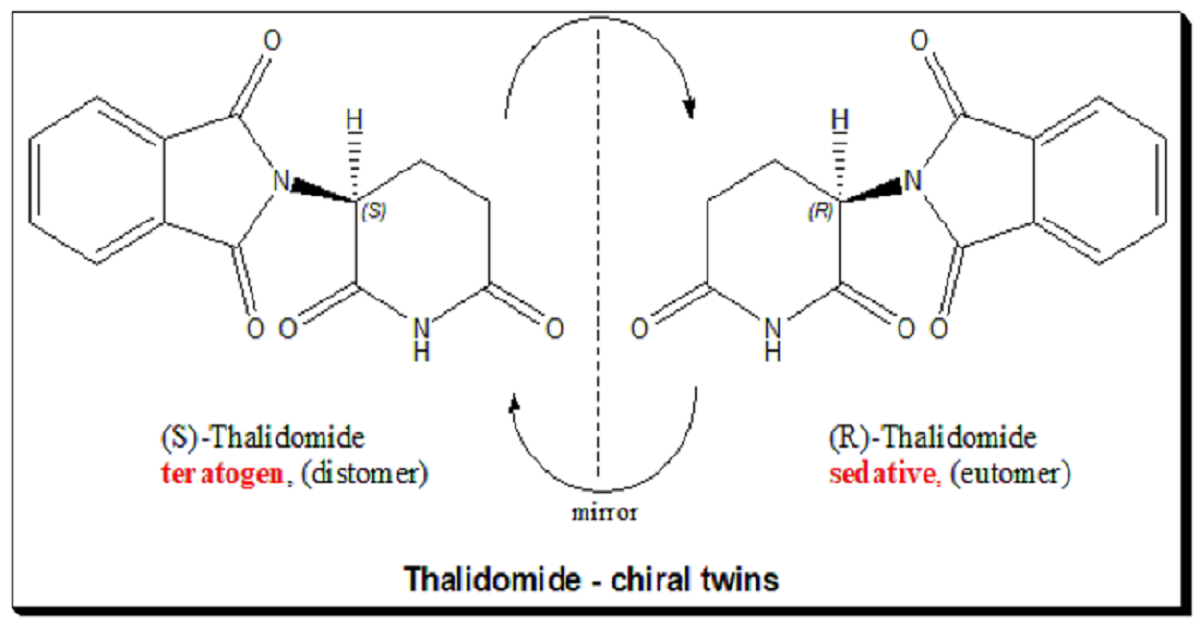
Thalidomide tragedy
The thalidomide tragedy illustrates the extreme consequences of administering a racemic drug with enantiomers having multiple effects. Thalidomide was marketed as a sedative and anti-nausea medication for pregnant women in the 1960s. Mothers who have taken the pill were giving birth to babies with a clinical condition referred to as “phocomelia” (deformed hands and limbs).Later, it was discovered that while the R-enantiomer had therapeutic effects, the S-enantiomer was teratogenic, causing severe birth defects. This tragedy highlighted the importance of understanding and controlling chirality in drug development. Today, thalidomide is used under strict controls for treating certain cancers and leprosy, with careful monitoring of its enantiomeric composition.
Independent Therapeutic Value
Propoxyphene is a classical example of a synthetic chiral drug where the enantiomers of a chiral drug exhibit desirable but different therapeutic activity. Example of such biological behavior is exemplified by some of the natural products as well (e.g., quinine and quinidine) with antimalarial and antiarrhythmic activity.
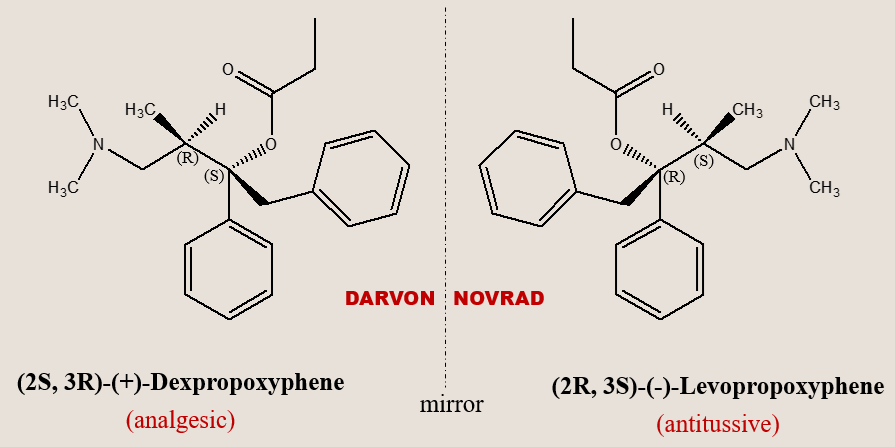
Enantiomers exhibiting independent therapeutic value
In certain instances, both isomers of a chiral drug can exhibit desirable but distinct therapeutic effects. A notable example is propoxyphene. Dexpropoxyphene serves as an analgesic, whereas its chiral counterpart, levopropoxyphene, lacks analgesic properties but functions as an antitussive. It is interesting to note that their mirror-image relationship is mirrored in their reversed trade names (DARVON and NOVRAD), under which Eli Lilly Company, USA, markets the two drugs..

Chiral Switch: Case Studies (select)
Chiral switch, a re-engineering approach, has led to the remarketing of several racemic drugs as chiral-specific enantiomer products. The chiral switching strategy is how most blockbuster drugs have entered the market as enantiopure drugs. Select case studies are presented below.
Ibuprofen
Ibuprofen is a commonly used NSAID for pain relief, inflammation, and fever reduction. It exists as a racemic mixture of S-ibuprofen and R-ibuprofen. Research shows that S-ibuprofen is primarily responsible for the drug’s therapeutic effects, while R-ibuprofen is converted to S-ibuprofen in the body through enzymatic action (chiral inversion). The pharmacokinetics and pharmacodynamics of these enantiomers are crucial for understanding the drug’s efficacy and safety. Ibuprofen was the first NSAID class chiral drug to switch to the single-enantiomer version (enantiopure/Unichiral) in 1994.
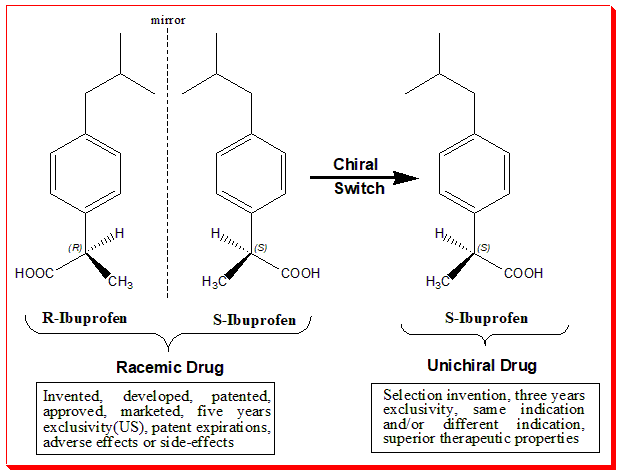
Chiral Switch
The chiral switch concept is illustrated in the diagram. This chiral switch is from (±)-ibuprofen to (S)-(+)-ibuprofen (dexibuprofen). The switch was done based on the fact that the (S)-ibuprofen, the eutomer, was over 100-fold more potent as an inhibitor of cycloxygenase-1 (COX-1) enzyme than (R)-ibuprofen.
Omeprazole
Omeprazole is a proton pump inhibitor used to treat conditions like gastroesophageal reflux disease (GERD). It is another chiral drug where one enantiomer, esomeprazole (the S-enantiomer), is marketed separately for its more consistent therapeutic effects. The development of esomeprazole from omeprazole, a chiral switch, illustrates the trend towards single-enantiomer drugs to enhance efficacy and reduce side effects.
Summary of Chiral Switch Strategy
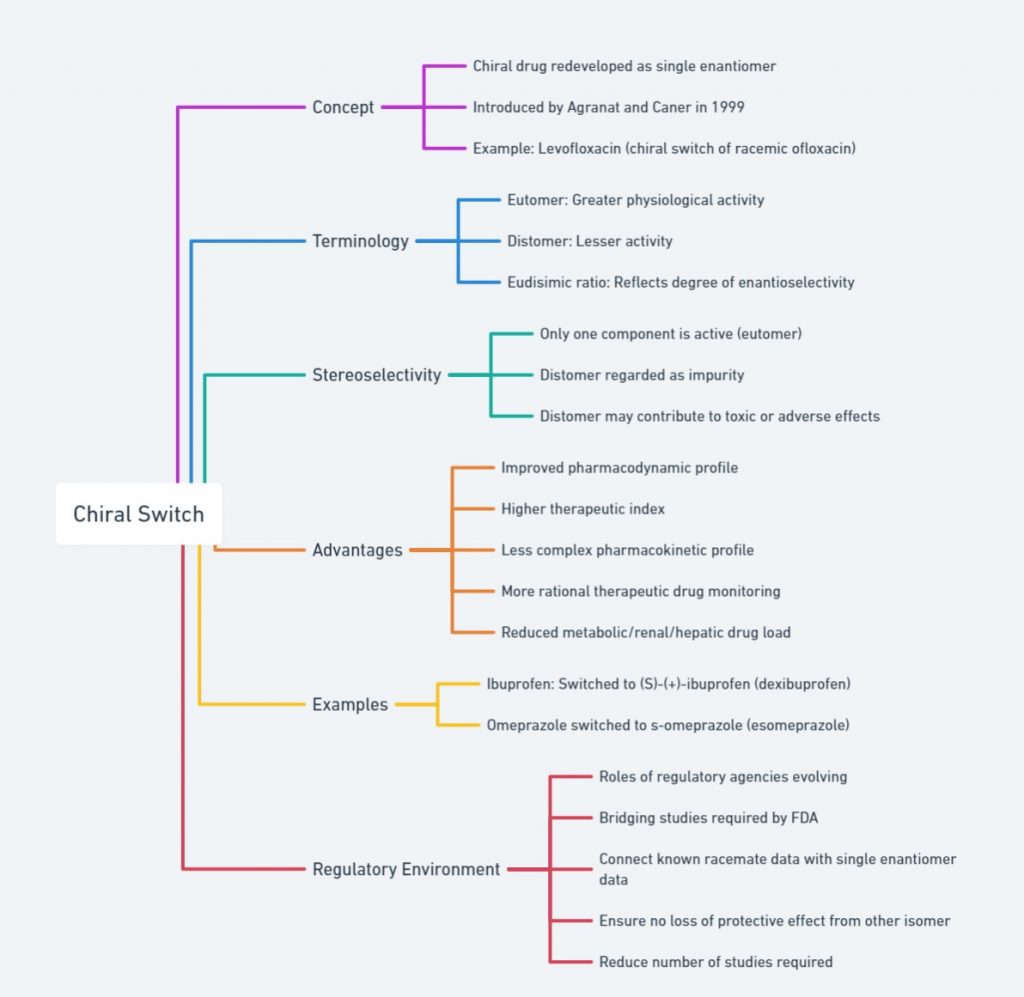
outlines chiral switching, where drugs are redeveloped as single enantiomers. Key concepts include the eutomer (active enantiomer), distomer (less active/inactive), and the eudismic ratio (degree of enantioselectivity). It highlights that the eutomer is typically active while the distomer may cause adverse effects. Advantages of chiral switches are improved pharmacodynamics, higher therapeutic indices, simpler pharmacokinetics, and more rational drug monitoring. Examples include the switches of ibuprofen to S-(+)-ibuprofen and omeprazole to S-omeprazole. The regulatory environment involves FDA bridging studies and reducing required studies.
Challenges in Separating and Synthesizing Enantiomers
One of the major challenges in pharmaceutical chemistry is the separation and synthesis of enantiomerically pure compounds. Racemic mixtures contain equal amounts of both enantiomers, but often only one enantiomer is therapeutically active. Achieving high enantiomeric purity is essential for drug safety and efficacy, but it can be technically demanding and costly.
Methods for Chiral Drug Synthesis
Traditional methods for the resolution of racemic mixtures involve crystallization or chromatography, but these can be inefficient. Asymmetric synthesis, where chiral catalysts or auxiliaries are used to preferentially produce one enantiomer, has become a more effective approach. Techniques such as asymmetric hydrogenation (The Nobel Prize in Chemistry 2001) and organocatalysis (The Nobel Prize in Chemistry 2021) have significantly advanced the field.
Advancements in Technology and Methods
Recent advancements in technology have improved the efficiency of chiral drug synthesis. Modern techniques in chiral chromatography, such as supercritical fluid chromatography (SFC), offer faster and more efficient separation of enantiomers. Biocatalysis, using enzymes to catalyze stereoselective reactions, represents a green and sustainable approach to synthesizing chiral drugs. These innovations are driving the development of safer and more effective pharmaceuticals.
A summary of the challenges and various methods employed for the separation and synthesis of enantiomers is given below.
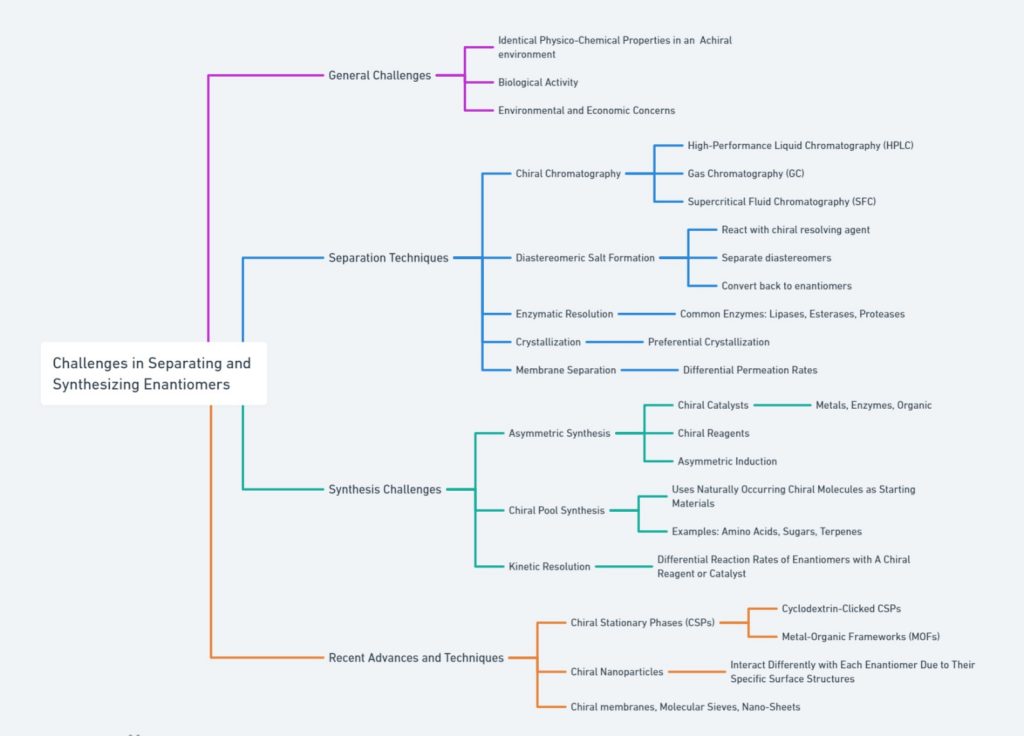
outlines the general challenges, separation techniques, synthesis challenges, and recent advances in enantiomer separation and synthesis. General challenges include identical properties in achiral environments, biological activity, and economic concerns. Separation techniques cover methods like HPLC, GC, SFC, diastereomeric salt formation, enzymatic resolution, crystallization, and membrane separation. Synthesis challenges involve chiral catalysts, reagents, asymmetric induction, chiral pool synthesis, and kinetic resolution. Recent advances highlight innovations such as cyclodextrin-clicked CSPs, MOFs, chiral nanoparticles, and advanced chiral membranes. This comprehensive view captures the complexities and latest developments in the field
Conclusion
Chirality is a fundamental aspect of pharmaceutical chemistry that significantly influences drug design, efficacy, and safety. Through understanding the role of enantiomers and developing advanced methods for synthesizing enantiomerically pure drugs, the pharmaceutical industry can create more effective and safer medications. The historical lessons from drugs like thalidomide, combined with modern advancements in chiral technology, highlight the critical importance of chirality in medicine. As research continues to evolve, the future of chiral pharmaceuticals promises to bring even more innovations and improvements to healthcare.
Suggested Further Readings
Lien Ai Nguyen, Hua He, and Chuong Pham-Huy, Chiral Drugs: An Overview, Int J Biomed Sci. 2006 Jun; 2(2): 85–100.
Hutt, A.J., Tan, S.C. Drug Chirality and its Clinical Significance. Drugs 52 (Suppl 5), 1–12 (1996). https://doi.org/10.2165/00003495-199600525-00003
Agranat, I., Caner, H., & Caldwell, J. (2002). Putting chirality to work: the strategy of chiral switches. Nature Reviews Drug Discovery, 1(10), 753–768. doi:10.1038/nrd915
https://en.wikipedia.org/wiki/Chiral_drugs
https://en.wikipedia.org/wiki/Chiral_switch
Francotte, Eric; Lindner, Wolfgang (2006). Chirality in drug research. Eric Francotte, W. Lindner. Weinheim: Wiley-VCH.
Brooks WH, Guida WC, Daniel KG. The significance of chirality in drug design and development, Curr Top Med Chem. 2011; 11(7): 760–770.doi: 10.2174/156802611795165098
Drayer, Dennis E (1986). “Pharmacodynamic and pharmacokinetic differences between drug enantiomers in humans: An overview”. Clinical Pharmacology and Therapeutics. 40 (2): 125–133. doi:10.1038/clpt.1986.150. ISSN 0009-9236. PMID 3731675. S2CID 33537650.
Ceramella, J.; Iacopetta, D.; Franchini, A.; De Luca, M.; Saturnino, C.; Andreu, I.; Sinicropi, M.S.; Catalano, A. A Look at the Importance of Chirality in Drug Activity: Some Significative Examples. Appl. Sci. 2022, 12, 10909. https://doi.org/10.3390/app122110909
Hutt, A. J.; Tan, S. C. (1996). “Drug Chirality and its Clinical Significance”. Drugs. 52 (Supplement 5): 1–12. doi:10.2165/00003495-199600525-00003. ISSN 0012-6667. PMID 8922553. S2CID 41802235.
Abram, M., Jakubiec, M., & Kamiński, K. (2019). Chirality as an important factor for the development of new antiepileptic drugs. ChemMedChem. doi:10.1002/cmdc.201900367
https://www.linkedin.com/pulse/why-chiral-separation-so-important-pharmaceuticals-pandey-v0ggc
https://remotecat.blogspot.com/2014/11/chiral-molecules-in-everyday-life-from.html
Rossino G, Robescu MS, Licastro E, Tedesco C, Martello I, Maffei L, Vincenti G, Bavaro T, Collina S. Biocatalysis: A smart and green tool for the preparation of chiral drugs. Chirality. 2022 Nov;34(11):1403-1418. doi: 10.1002/chir.23498. Epub 2022 Aug 5. PMID: 35929567; PMCID: PMC9805200.
Tang, J., Zhang, S., Lin, Y. et al. Engineering Cyclodextrin Clicked Chiral Stationary Phase for High-Efficiency Enantiomer Separation. Sci Rep 5, 11523 (2015). https://doi.org/10.1038/srep11523
Other Blogs on #ChiralityonFriday
1. Introduction to Chirality: Understanding the Basics
2. Molecular Handedness: How Chirality Shapes Molecules
3. Chirality in Nature: From DNA to Snail Shells
4. The Chemistry of Taste and Smell: How Chirality Affects Senses
5. Chiral Chemistry in Everyday Life: Hidden Handedness Around Us
6. Chirality in Pharmaceuticals: The Impact of Molecular Handedness on Medicine
7. https://chiralpedia.com/blog/chirality-in-materials-science-designing-with-handedness/
8. Chirality in Space: Cosmic Asymmetry and the Origins of Life
9. The Future of Chirality: Innovations and Emerging Trends
10. Chirality and You: Understanding and Appreciating Molecular Handedness


Sir thank you for sharing most valuable and vivid content on the importance of chirality with regard to drug actions, I was really benefitted after going through your blog. I have already shared your blog chiralpedia.com with my students, I feel it will be of much help for the students to learn and understand the concept from your site, thank you sir
Dear Professor Rajini,
Glad to note that you find the blog useful and informative for the student community. Happy that the basic purpose of chiralpedia is to served. Tell your faculty and students to take tour of chiralpedia !!!
Chirally yours,
Valliappan