Synopsis
Chiral drugs, those fascinating chemical compounds existing as mirror-image pairs or enantiomers, exhibit distinct biological properties. This post provides a comprehensive overview of chiral drugs, presenting key concepts through a visually engaging mind maps. It covers chirality’s historical roots and stereochemical nomenclature, delves into the specialized discipline of chiral pharmacology, and presents drug toxicity case studies. Additionally, it explores unichiral drugs, the importance of chiral purity, and the tools used for measuring it. Designed with clarity and depth in mind, this content invites readers to explore the fascinating world of chiral drugs and their critical role in modern medicine. <To explore this intriguing topic further, be sure to check out the “Further Reading” section for expert insights>
Core Mind Map
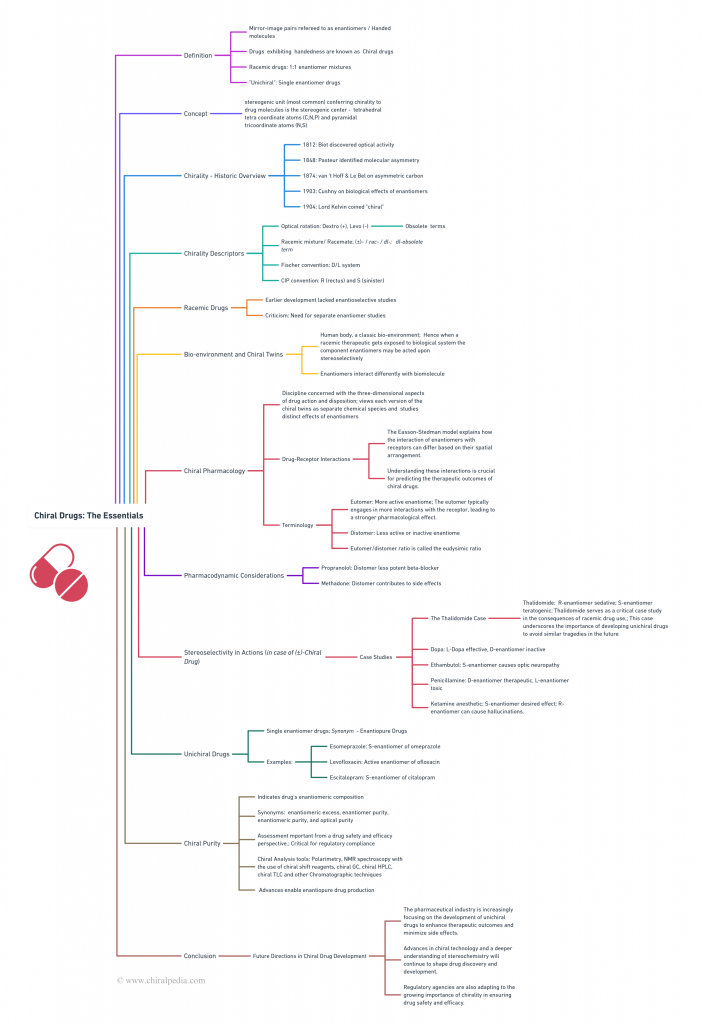
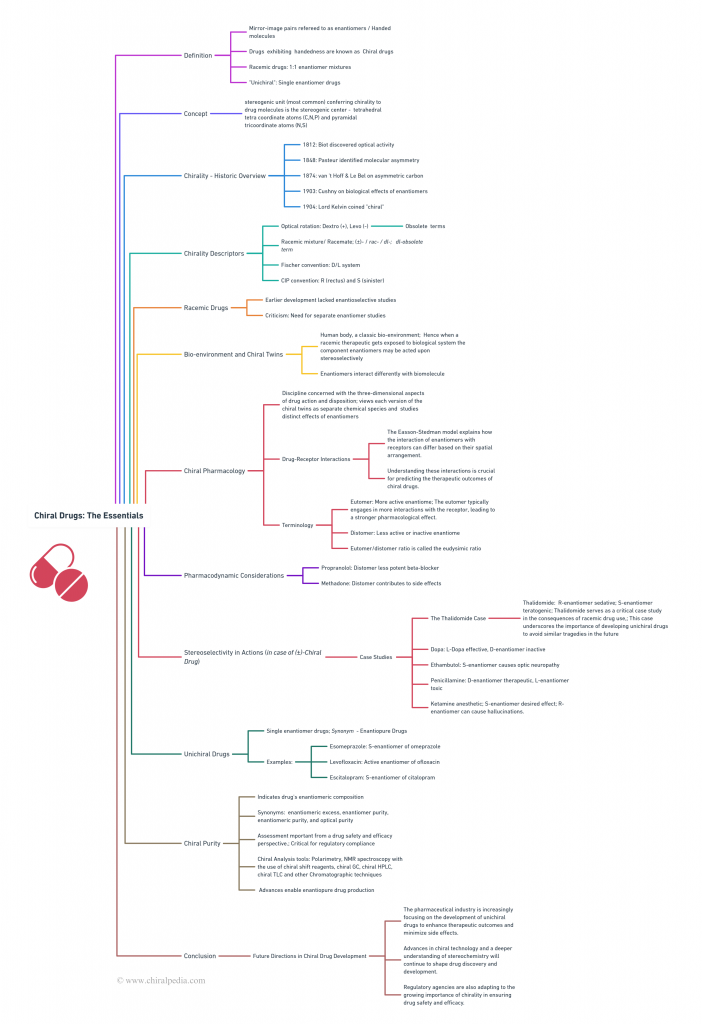
Explore the twisted tale of chiral drugs through our captivating Concept Map! This visual journey elegantly weaves together the historical roots, the intricacies of chiral and racemic drugs, and the chiral nomenclature system. Dive into the bio-environment and discover the unique behaviors of these fascinating compounds. Designed for clarity and ease of understanding, this overview provides a comprehensive insight into the essentials of chiral drugs.
But there’s more – venture into the specialized realm of “chiral pharmacology”. Uncover concepts like chiral discrimination, the Easson-Stedman model, and stereochemically engineered drug toxicity case studies. Learn about the significance of chiral purity and the tools used to measure it, exploring unichiral drugs and their critical role in modern medicine. This visual exploration promises to make your journey through the world of chiral drugs both enlightening and visually engaging!
To enhance readability and visual comfort, the main idea map “Chiral Drugs: The Essentials” has been thoughtfully divided into two parts: “Chiral Drugs: The Essentials 1” and “Chiral Drugs: The Essentials 2 – Chiral Pharmacology.” This approach aims to provide a smoother journey through the intricate world of chiral pharmaceuticals, ensuring you can navigate these complex concepts with ease
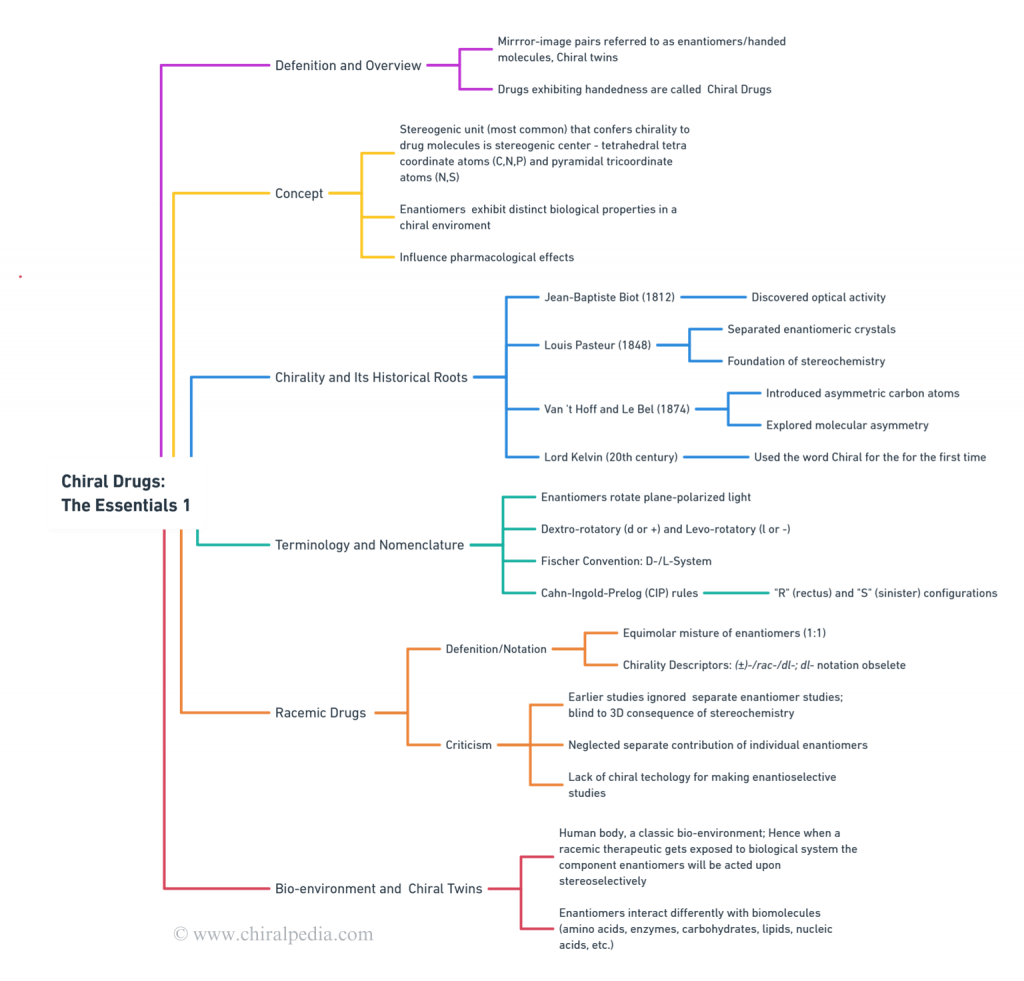
Discover the intricate world of chirality through our detailed Concept Map. This visual presentation elegantly captures the historical roots, nuances of chiral and racemic drugs, the chiral nomenclature system, bio-environment, and the distinct behavior of chiral drugs. Designed for clarity and ease of understanding, this overview offers a comprehensive insight into the essentials of chiral drugs
Chiral Pharmacology
Now, let’s embark on an exciting journey into the captivating world of Chiral Pharmacology, also known as Stereo-Pharmacology. Dive into the fascinating concepts through our detailed and engaging concept maps, and discover the intricate science behind the unique behavior of chiral drugs.
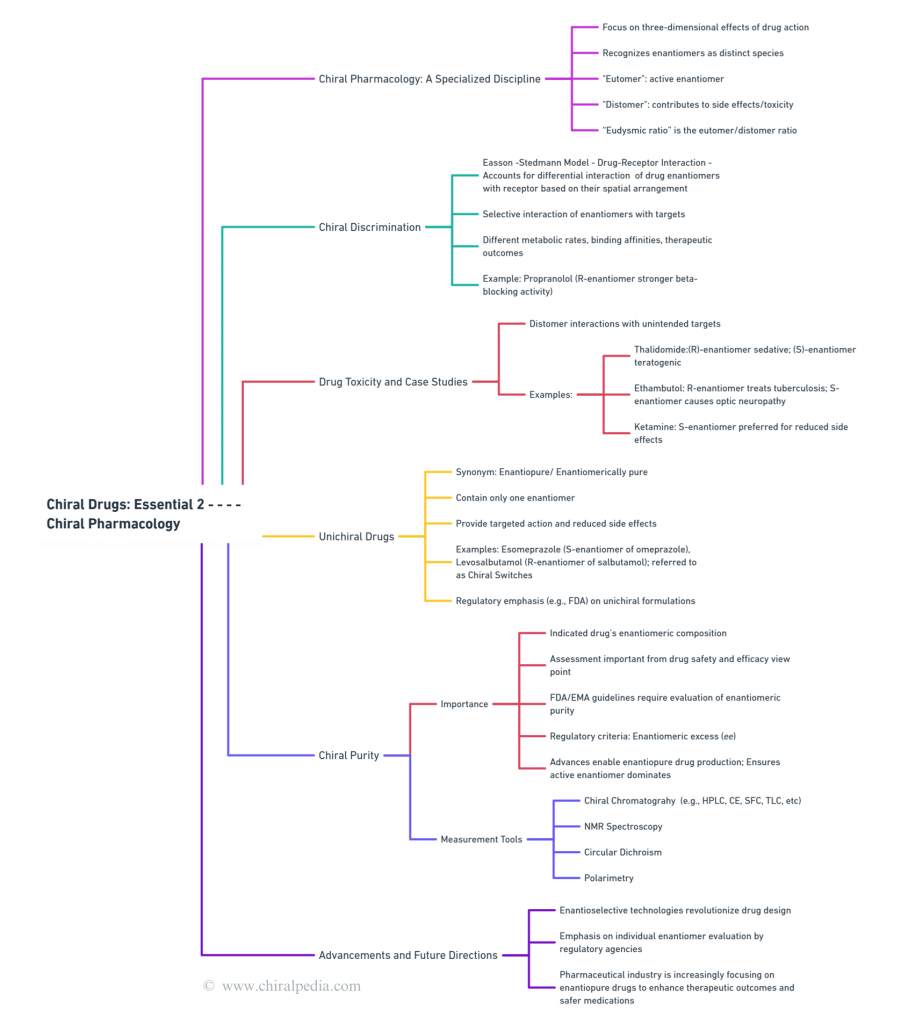
These maps will guide you through the complexities of chiral discrimination, the Easson-Stedman model, and specialized drug toxicity case studies. Uncover the importance of chiral purity and the innovative tools used to measure it. Get ready to explore the pivotal role of unichiral drugs in modern medicine with clarity and depth
Conclusion
Chiral drugs are a fascinating and essential aspect of modern pharmaceuticals, offering unique biological properties and critical advantages in medication efficacy and safety. By understanding the historical roots of chirality, mastering stereochemical nomenclature, and exploring chiral pharmacology, we gain valuable insights into the complexities and benefits of these compounds. As the pharmaceutical industry continues to advance, the importance of chiral purity and the tools used to measure it cannot be overstated. This exploration of chiral drugs provides a comprehensive foundation for appreciating their critical role in medicine today.
Future Direction
Looking ahead, the field of chiral pharmacology holds immense potential for further breakthroughs and innovations. Future research may focus on:
Developing New Unichiral Drugs: Continuing to explore and create unichiral drugs with enhanced efficacy and reduced side effects.
Advancing Analytical Techniques: Improving tools and methods for measuring chiral purity to ensure the highest standards in drug development.
Personalized Medicine: Tailoring chiral drug therapies to individual patient needs, leveraging genetic and biochemical profiling.
Environmental Impact: Investigating the ecological effects of chiral drugs and finding sustainable solutions to mitigate any negative impacts.
Regulatory Standards: Establishing and refining regulatory guidelines to ensure consistent and safe production of chiral drugs.
By staying at the forefront of these developments, we can continue to unlock the full potential of chiral drugs, ultimately improving patient outcomes and advancing the field of pharmaceutical science.
Further Reading
Bassindale, A (1984). The Third Dimension in Organic Chemistry. New York: John Wiley, New York. ISBN 047190189X.
Shou-jiao Peng et al. (2024). Chiral drugs: Sources, absolute configuration identification, pharmacological applications, and future research trends. LabMed Discovery, 1, 1. https://doi.org/10.1016/j.lmd.2024.100008
Yaoyu Zhou et al. (2018), Chiral pharmaceuticals: Environment sources, potential human health impacts, remediation technologies and future perspective, Environment International, 121,1, 523-537. https://doi.org/10.1016/j.envint.2018.09.041
Nguyen LA, He H, Pham-Huy C. (2006). Chiral drugs: an overview. Int J Biomed Sci. Jun;2(2):85-100. PMID: 23674971; PMCID: PMC3614593.
Gal, Joseph; Lindner, wolfgang (2006). “Chiral drugs from a historical point of view”. In Francotte, Eric (ed.). Chirality in drug research. Germany: Wiley-VCH Verlag GmbH & Co. pp. 3–26. ISBN 3-527-31076-2.
Gal, J (1998). “Problems of stereochemical nomenclature and terminology. 1. The homochiral controversy. Its nature, origins, and a proposed solution”. Enantiomer. 3: 263–273.
Jamali, F.; Mehvar, R.; Pasutto, F.M. (1989). “Enantioselective Aspects of Drug Action and Disposition: Therapeutic Pitfalls”. Journal of Pharmaceutical Sciences. 78 (9): 695–715. doi:10.1002/jps.2600780902. ISSN 0022-3549. PMID 2685226.
Williams, Kenneth M. (1991), Molecular Asymmetry and Its Pharmacological Consequences, Advances in Pharmacology, vol. 22, Elsevier, pp. 57–135, doi:10.1016/s1054-3589(08)60033-2, ISBN 978-0-12-032922-9, PMID 1958505, retrieved 2021-06-03
Crossley, Roger (1992). “The relevance of chirality to the study of biological activity”. Tetrahedron. 48 (38): 8155–8178. doi:10.1016/s0040-4020(01)80486-5. ISSN 0040-4020.
Gross, Michael (1990), Chapter 34. Significance of Drug Stereochemistry in Modern Pharmaceutical Research and Development, Annual Reports in Medicinal Chemistry, vol. 25, Elsevier, pp. 323–331, doi:10.1016/s0065-7743(08)61610-3, ISBN 978-0-12-040525-1, retrieved 2021-06-03
Decamp, W (1989). “The FDA perspective on the development of stereoisomers”. Chirality. 1 (1): 2–6. doi:10.1002/chir.530010103. PMID 2642032.
Ariens, E. J. (1984). “Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology”. European Journal of Clinical Pharmacology. 26 (6): 663–668. doi:10.1007/bf00541922. ISSN 0031-6970. PMID 6092093. S2CID 30916093.
Ariëns, Everardus J. (1987). “Stereochemistry in the analysis of drug-action. Part II”. Medicinal Research Reviews. 7 (3): 367–387. doi:10.1002/med.2610070305. ISSN 0198-6325. PMID 3041134. S2CID 31403941.
Ariëns, E.J (1987). “Implications of the Neglect of Stereochemistry in Pharmacokinetics and Clinical Pharmacology”. Br. J. Clin. Pharmacol. Ther. 21 (10): 827–829. doi:10.1177/106002808702101013. PMID 3322758. S2CID 23007083.
Ariëns, Everd J. (1987). “Implications of the Neglect of Stereochemistry in Pharmacokinetics and Clinical Pharmacology”. Drug Intelligence & Clinical Pharmacy. 21 (10): 827–829. doi:10.1177/106002808702101013. ISSN 0012-6578. PMID 3322758. S2CID 23007083.
Ariëns, E. J. (1989). “Stereoselectivity in the Action of Drugs”. Pharmacology & Toxicology. 64 (4): 319–320. doi:10.1111/j.1600-0773.1989.tb00655.x. ISSN 0901-9928. PMID 2748538.
Ariëns, E.J. (1986). “Chirality in bioactive agents and its pitfalls”. Trends in Pharmacological Sciences. 7: 200–205. doi:10.1016/0165-6147(86)90313-5. ISSN 0165-6147.
Caldwell, John (1995). “Chiral Pharmacology and the regulation of new drugs”. Chemistry and Industry. 6: 176–179.
Ariëns, Everhardus J.; Wuis, Eveline W.; Veringa, Eric J. (1988). “Stereoselectivity of bioactive xenobiotics”. Biochemical Pharmacology. 37 (1): 9–18. doi:10.1016/0006-2952(88)90749-6. ISSN 0006-2952. PMID 3276322.
Wainer, Irving W. (1992-09-01). “Three-dimensional view of pharmacology”. American Journal of Health-System Pharmacy. 49 (9_Suppl): S4 – S8. doi:10.1093/ajhp/49.9_suppl_1.s4. ISSN 1079-2082. PMID 1530004.
Drayer, Dennis E (1986). “Pharmacodynamic and pharmacokinetic differences between drug enantiomers in humans: An overview”. Clinical Pharmacology and Therapeutics. 40 (2): 125–133. doi:10.1038/clpt.1986.150. ISSN 0009-9236. PMID 3731675. S2CID 33537650. Scott, Andrew K. (1990).
“Stereoisomers in Clinical Pharmacology”. Drug Information Journal. 24 (1): 121–123. doi:10.1177/009286159002400123. ISSN 0092-8615. S2CID 72095771.
Lehmann, Pedro A.F. (1986). “Stereoisomerism and drug action”. Trends in Pharmacological Sciences. 7: 281–285. doi:10.1016/0165-6147(86)90353-6. ISSN 0165-6147.
Ariëns, E.J. (1990), “Racemic Therapeutics—Problems all Along the Line”, Chirality in Drug Design and Synthesis, Elsevier, pp. 29–43, doi:10.1016/b978-0-12-136670-4.50008-4, ISBN 978-0-12-136670-4, retrieved 2021-06-03
Hutt, A. J.; Tan, S. C. (1996). “Drug Chirality and its Clinical Significance”. Drugs. 52 (Supplement 5): 1–12. doi:10.2165/00003495-199600525-00003. ISSN 0012-6667. PMID 8922553. S2CID 41802235.
Simonyi, Miklós (1984). “On chiral drug action”. Medicinal Research Reviews. 4 (3): 359–413. doi:10.1002/med.2610040304. ISSN 0198-6325. PMID 6087043. S2CID 38829275.
Williams, Kenneth; Lee, Edmund (1985). “Importance of Drug Enantiomers in Clinical Pharmacology”. Drugs. 30 (4): 333–354. doi:10.2165/00003495-198530040-00003. ISSN 0012-6667. PMID 3905334. S2CID 23999344.
Ariëns, Everardus J. (1986). “Stereochemistry: A source of problems in medicinal chemistry”. Medicinal Research Reviews. 6 (4): 451–466. doi:10.1002/med.2610060404. ISSN 0198-6325. PMID 3534485. S2CID 36115871.
Baldwin, J.J.; Abrams, W. B (1988). Wainer, I.W.; Drayer, D.E. (eds.). Drug Stereochemistry, Analytical Methods and Pharmacology. New York: Marcel Dekker, New York. pp. 311–356.
Williams, K. M (1990). Problems and wonder of chiral molecules. Budapest: Akademiai Kiado, Budapest. pp. 181–204. ISBN 963-05-5881-5.
Scott, Andrew K. (1993). “Stereoisomers and Drug Toxicity”. Drug Safety. 8 (2): 149–159. doi:10.2165/00002018-199308020-00005. ISSN 0114-5916. PMID 8452656. S2CID 20426452.
Powell, J, R. (1988). Drug stereochemistry. Analytical methods and pharmacology. New York: Marcel Dekker, New York. pp. 245–270.
Wright, Matthew R.; Jamali, Fakhreddin (1993). “Methods for the analysis of enantiomers of racemic drugs application to pharmacological and pharmacokinetic studies”. Journal of Pharmacological and Toxicological Methods. 29 (1): 1–9. doi:10.1016/1056-8719(93)90044-f. ISSN 1056-8719. PMID 8481555.
Ariens, E.J (1989). Chiral Separations by HPLC. Chichester: Ellis Horwood, Chichester. pp. 31–68.
Hyneck, M (1990). Chirality in Drug Design and Synthesis. New York: Academic Press, New York. pp. 1–28.
Blessington, Bernard (1997). The impact of stereochemistry on drug development and use. New York: John Wiley & Sons, New York. pp. 235–261. ISBN 0-471-59644-2.
Ernest, L Eliel; Samuel H, Wilen (1990). “Misuse of homochiral”. Chemical & Engineering News. 10: 2.
Cayen, Mitchell N. (1991). “Racemic mixtures and single stereoisomers: Industrial concerns and issues in drug development”. Chirality. 3 (2): 94–98. doi:10.1002/chir.530030203. ISSN 0899-0042.
Caldwell, John (1996). “Importance of stereospecific bionalytical monitoring in drug development”. Journal of Chromatography A. 719 (1): 3–13. doi:10.1016/0021-9673(95)00465-3. ISSN 0021-9673. PMID 8589835.
Campbell, D.B. (1990). “The development of chiral drugs”. Acta Pharm. Nord. 2 (3): 217–226. PMID 2200432.
https://en.wikipedia.org/wiki/Chiral_drugs
Allenmark, Stig G. (1988). Chromatographic enantioseparation: methods and applications. Chichester: Ellis Horwood. pp. 27–40. ISBN 9780131329454.