Introduction
Chirality, or molecular handedness, is a fascinating aspect of chemistry that plays a crucial role in the structure and function of many substances we encounter in our daily lives. From the food we eat to the cleaning products we use, chirality influences the properties and effectiveness of numerous everyday items. This blog explores the hidden handedness of chiral chemistry in household products, food and beverages, and other common examples, highlighting their significance and impact. [For explanation on the stereochemical notations/descriptors used in the blog please consult our earlier blog <https://chiralpedia.com/blog/d-l-system-naming-the-left-or-right-hand-side/>, <https://chiralpedia.com/blog/naming-enantiomers-the-r-s-system/>, and <https://chiralpedia.com/blog/introduction-to-chirality-understanding-the-basics/>.
Household Products
Personal Care Products
Chirality is also prevalent in personal care products, including cosmetics (skin care) and hygiene items. Many active ingredients in these products are chiral, and the specific enantiomers used can significantly impact their effectiveness.
Dental Care
For instance, the chiral compound menthol, found in toothpaste and mouthwash, provides a cooling sensation. The l-enantiomer of menthol is more effective at activating cold-sensitive receptors in the mouth, enhancing the product’s sensory experience. Similarly, chiral compounds in shampoos and lotions can improve their moisturizing and soothing properties.
Skin Care
Human skin is also chiral; therefore, it responds best to skin care products with chirally-correct ingredients and formulations, more specifically the correct isomer of the ingredient. For example, vitamin C contains two isomers (L and D), one produces great benefits for the skin (L-ascorbic), and another produces little to no effect (D-ascorbate). Through the science of chirality, these two isomers may be identified and separated, producing only the isomer with results.
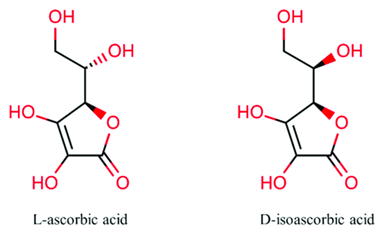
Understanding and utilizing the correct chiral form of vitamin C is essential for maximizing the efficacy of skincare products.
In the context of skincare products, chirality plays a crucial role in the effectiveness of vitamin C. L-ascorbic acid is the active form of vitamin C commonly used in skincare formulations due to its superior antioxidant properties, aiding in collagen synthesis and providing photoprotection. Conversely, D-isoascorbic acid, though structurally similar, does not offer the same benefits for skin health.

Similarly, all the amino acids (L-) in human cells are left-handed; no protein in the body will contain a right-handed amino acid. Sugars (D-), however, are right-handed. Ingredients exfoliating the skin such as malic, lactic and ascorbic acid use L-isomers. For instance, a product containing lactic acid, for example, may not deliver the maximum benefits associated with lactic – cell turnover, reduced aging and moisturizing – if it contains both the (L-) and (D-) forms. It is the (L-) form giving lactic its rejuvenation characteristics.
On the other hand, ingredients strengthening, protecting and hydrating the skin, like tocopherol, beta glucosamine and fructan use D-isomers. However, there are several chiral molecules requiring equal amounts of the left- and right-hand enantiomers. These are known as racemic mixtures and include ingredients like retinals and glycolic acid. This is why knowing ingredient levels and what was used in the base formula is important.
Cleaning Agents and Detergents
Chiral surfactants are essential components of many cleaning agents and detergents. These molecules have a hydrophilic (water-attracting) end and a hydrophobic (water-repelling) end, allowing them to break down grease and dirt effectively. Chiral surfactants, such as those derived from amino acids or sugars, often exhibit enhanced cleaning properties compared to their achiral counterparts. For example, chiral surfactants can form micelles with better-defined structures, leading to more efficient emulsification of oils and removal of stains.
Pharmaceuticals
Our household medicine cabinets often contain chiral drugs, highlighting the importance of chirality in pharmaceuticals. The effectiveness and safety of many medications depend on their chirality. For example, ibuprofen, a common pain reliever, is chiral, and its S-enantiomer is primarily responsible for its therapeutic effects. Understanding the role of chirality in pharmaceuticals helps ensure that the drugs we use are both safe and effective. [This will be dealt in detail in the forthcoming blog <Chirality in Pharmaceuticals: The Impact of Molecular Handedness on Medicine>
Chirality in Agrochemicals
The phenomenon of enantioselectivity in biological activity is universal. It is also observed in agrochemicals that act on living organisms – plants, insects, and fungi. The overwhelming majority of chiral agrochemicals are are marketed as racemates. In some countries use of racemates has been severely curtailed. It is interesting to note that chiral herbicides flamprop-isopropyl and carbetamide are developed as the pure R-enantiomers. For instance, deltamethrin, a pyrethroid insecticide, has three chiral centers and is sold as a single (R,R,S)-enantiomer, one of the eight possible stereoisomers. Similarly the chiral fungicide (R)-metaxalyl is shown to be about 1000 times more active than the (S)- enantiomer in invitro studies.
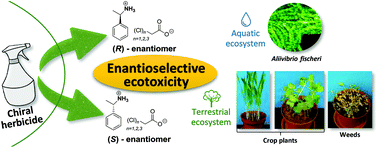
Source https://pubs.rsc.org/en/content/articlelanding/2022/gc/d1gc03970a
Need to change the approach to the safe use of herbicides by developing chiral and environmentally friendly formulations.
Most of the commercial chiral herbicides are used as racemic mixtures, whereas the use of their optically active forms may be more effective and environmentally safer. For this reason, it is important to evaluate the ecotoxicity of each enantiomer separately because a seemingly inactive enantiomer may unintentionally be toxic to non-target organisms.
Chirality in Food and Beverages
Flavor Compounds
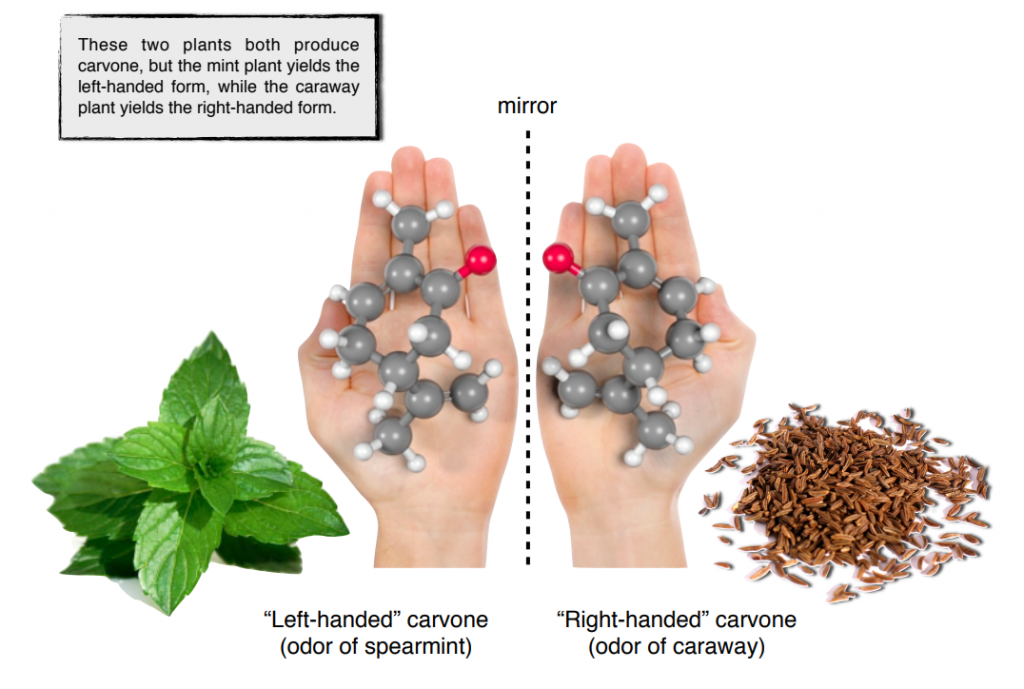
Chirality plays a crucial role in the flavor and aroma of food and beverages. Many flavor compounds are chiral, and their enantiomers can have distinct tastes and smells. For example, the chiral molecule carvone exists as two enantiomers: R-carvone, which smells like spearmint and S-carvone, which smells like caraway.
Similarly, limonene, another chiral molecule, has a D-enantiomer that smells like oranges and an L-enantiomer that smells like lemons. These differences in sensory perception are due to the way each enantiomer interacts with olfactory receptors in the nose.
Nutritional Supplements
Chirality is also important in nutritional supplements, particularly for amino acids and vitamins. Many essential nutrients are chiral, and their bioavailability and effectiveness can depend on their chirality. For instance, the L-enantiomers of amino acids are more readily absorbed and utilized by the body compared to their D-enantiomers. This is because enzymes involved in protein synthesis and metabolism are specific to the L-form.

All amino acids except glycine are chiral because they all contain at least one chiral center
The image displays the general structure of an amino acid, highlighting its key functional groups: an amino group (green), a carboxyl group (yellow), and a variable side chain (R group, red). The central carbon (alpha carbon) to which these groups are attached is a chiral center (except in glycine, where the R group is a hydrogen atom, making it achiral). The chirality of amino acids is fundamental in biochemistry as it determines the spatial arrangement of the molecules, influencing their interactions and functions in biological systems. Most naturally occurring amino acids are in the L-configuration, which is crucial for protein synthesis and proper enzymatic activity. The specific chirality of amino acids affects protein folding, enzyme specificity, and receptor binding, ultimately impacting cellular processes and organismal physiology. Understanding amino acid chirality is essential in drug design, biotechnology, and the study of metabolic pathways.
Similarly, chiral vitamins, such as vitamin E, have enantiomers with different biological activities, impacting their nutritional value.

The RRR alpha-tocopherol form of vitamin E
Alcoholic Beverages
Chiral compounds are present in alcoholic beverages, influencing their flavor and aging processes. For example, certain chiral alcohols and esters contribute to the complex aromas of wine and spirits. The chirality of these compounds affects their interaction with other molecules, altering the overall sensory profile of the beverage. In winemaking, the chirality of compounds like tartaric acid can influence the stability and taste of the wine. Understanding the role of chirality in alcoholic beverages helps producers create more refined and enjoyable products.
Everyday Examples and their Significance
Smells and Fragrances
Chiral molecules are key components of many perfumes and air fresheners. The chirality of fragrance molecules can greatly influence their scent perception. For example, the chiral molecule limonene has enantiomers with distinct smells, as mentioned earlier <https://chiralpedia.com/blog/the-chemistry-of-taste-and-smell-how-chirality-affects-senses/>. This principle is applied in the fragrance industry to create perfumes with specific scent profiles. The ability to control the chirality of fragrance molecules allows for the design of products that cater to diverse consumer preferences.

Fragrance Design
Fragrance creation conceptually employs an olfactive pyramid, where different notes are layered similarly to a fine art piece. A fragrance may consist of hundreds of ingredients, blended harmoniously like a musical symphony. These notes are categorized into top, middle, and base notes. Even small alterations in the structure, stereochemistry, shape, and chirality of a fragrance ingredient or scent molecule can significantly affect its aroma and appeal.
Chiral Materials and their Applications
Chirality is not limited to molecules; it can also manifest in materials. Chiral materials, such as certain polymers, are used in a variety of consumer products. For instance, chiral polymers can be used in packaging materials to improve their barrier properties and durability. These materials can also be designed to interact selectively with specific molecules, enhancing their performance in applications like filtration, chiral separation, and drug delivery systems. The role of chirality in material science is expanding, leading to the development of innovative products with improved functionalities. This will be discussed in the forthcoming blog on <Chirality in Materials Science: Designing with Handedness>.
Conclusion
Chirality is a fundamental aspect of chemistry that significantly impacts our everyday lives. From household products and personal care items to food and beverages, chiral compounds play crucial roles in enhancing the effectiveness, safety, and sensory experiences of these products. Recognizing the hidden handedness around us helps us appreciate the complexity and importance of chirality in our daily routines. As research in chiral chemistry continues to advance, we can expect further innovations and improvements in consumer products, driven by a deeper understanding of molecular handedness.
Suggested Further Readings
Chirotechnology: Industrial synthesis of optically active compounds, Roger A. Sheldon, New York: Marcel Dekker, Inc., 1993, xvii + 423 pages, ISBN: 0-8247-9143-6. https://doi.org/10.1002/chir.530060109
https://chempics.wordpress.com/tag/menthol
Gusain P, Ohki S, Hoshino K, Tsujino Y, Shimokawa N, Takagi M. Chirality-Dependent Interaction of d- and l-Menthol with Biomembrane Models. Membranes (Basel). 2017 Dec 15;7(4):69. doi: 10.3390/membranes7040069. PMID: 29244740; PMCID: PMC5746828.
https://remotecat.blogspot.com/2014/11/chiral-molecules-in-everyday-life-from.html
https://www.food.actapol.net/pub/2_1_2006.pdf
https://redmethod.com/a/info/blog/how-the-science-of-chirality-impacts-your-skin
https://www.arcona.com/blog/what-is-chirally-correct-skincare-and-why-is-it-better-for-you/
https://www.acsh.org/news/2018/08/01/carvone-one-molecule-two-different-scents-and-flavors-13255
Silas W. Smith , Chiral Toxicology: It’s the Same Thing…Only Different, Toxicological Sciences, Volume 110, Issue 1, July 2009, Pages 4–30, https://doi.org/10.1093/toxsci/kfp097
Manolescu B, Atanasiu V, Cercasov C, Stoian I, Oprea E, Buşu C. So many options but one choice: the human body prefers alpha-tocopherol. A matter of stereochemistry. J Med Life. 2008 Oct-Dec;1(4):376-82. PMID: 20108516; PMCID: PMC5654212.
https://socratic.org/questions/why-are-amino-acids-chiral
Mohd Zaffarin AS, Ng SF, Ng MH, Hassan H, Alias E. Pharmacology and Pharmacokinetics of Vitamin E: Nanoformulations to Enhance Bioavailability. Int J Nanomedicine. 2020 Dec 8;15:9961-9974. doi: 10.2147/IJN.S276355. PMID: 33324057; PMCID: PMC7733471.
Regina Brigelius-Flohe´, Bioactivity of vitamin E, Nutrition Research Reviews (2006), 19, 174–186. doi: 10.1017/NRR2006125
https://lpi.oregonstate.edu/mic/vitamins/vitamin-E
http://www.leffingwell.com/Chem_Spec_Mag-1.pdf
https://pubs.acs.org/doi/10.1021/bk-2019-1321.ch001
Engel, K.-H. (2020). Chirality- an important phenomenon regarding biosynthesis, perception and authenticity of flavour compounds. Journal of Agricultural and Food Chemistry. doi:10.1021/acs.jafc.0c01512
Adawy, A. Functional Chirality: From Small Molecules to Supramolecular Assemblies. Symmetry 2022, 14, 292. https://doi.org/10.3390/sym14020292https://www.dgaryyoung.com/blog/2011/part-18-finding-pure-essential-oils/
https://www.musechem.com/blog/the-application-of-chiral-molecules-in-our-lives
Other Blogs on #ChiralityonFriday
1. Introduction to Chirality: Understanding the Basics
2. Molecular Handedness: How Chirality Shapes Molecules
3. Chirality in Nature: From DNA to Snail Shells
4. The Chemistry of Taste and Smell: How Chirality Affects Senses
5. Chiral Chemistry in Everyday Life: Hidden Handedness Around Us
7. https://chiralpedia.com/blog/chirality-in-materials-science-designing-with-handedness/
8. Chirality in Space: Cosmic Asymmetry and the Origins of Life
9. The Future of Chirality: Innovations and Emerging Trends
10. Chirality and You: Understanding and Appreciating Molecular Handedness

