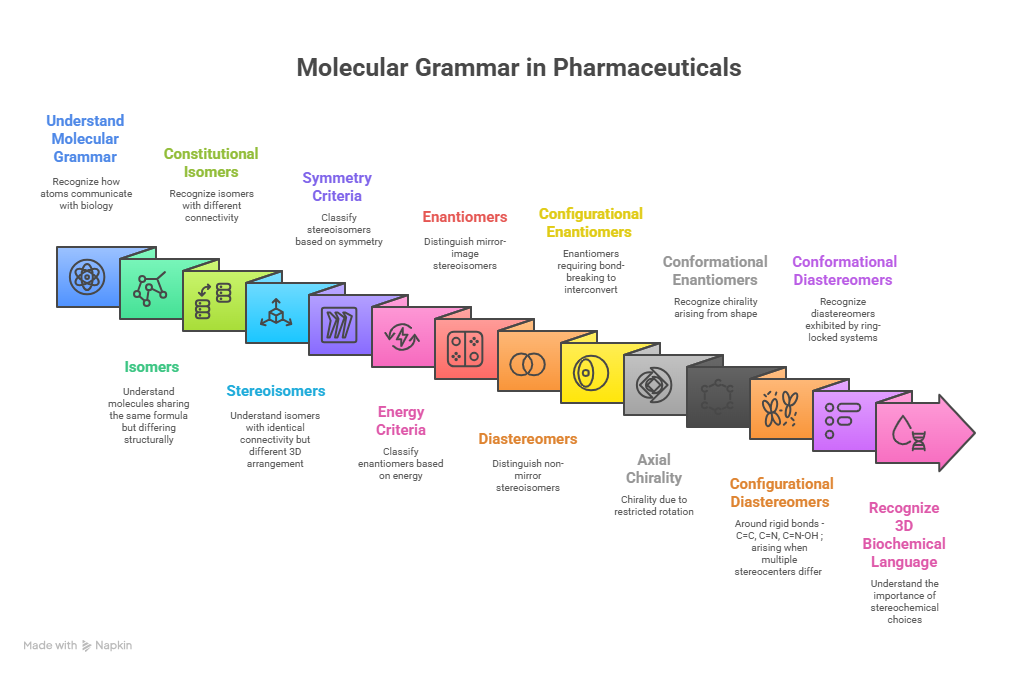
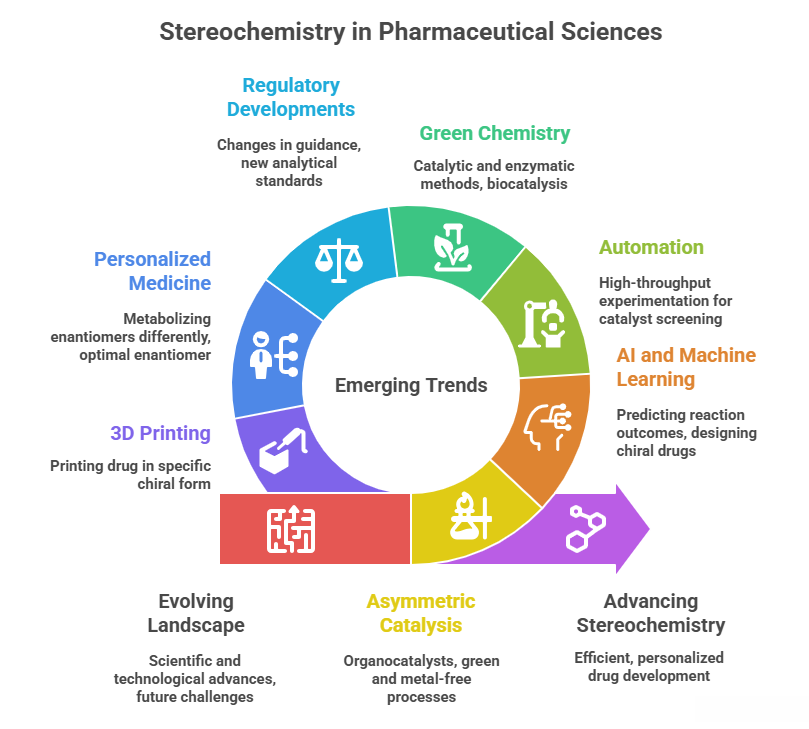
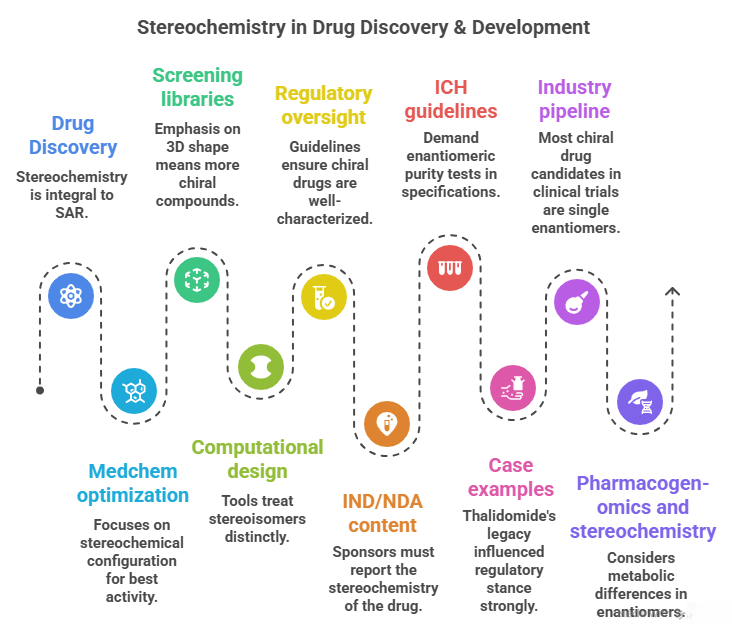
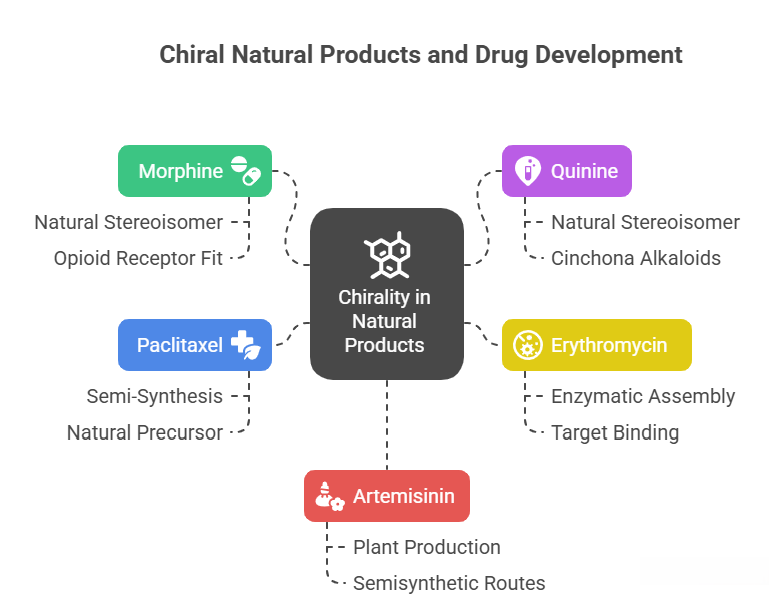
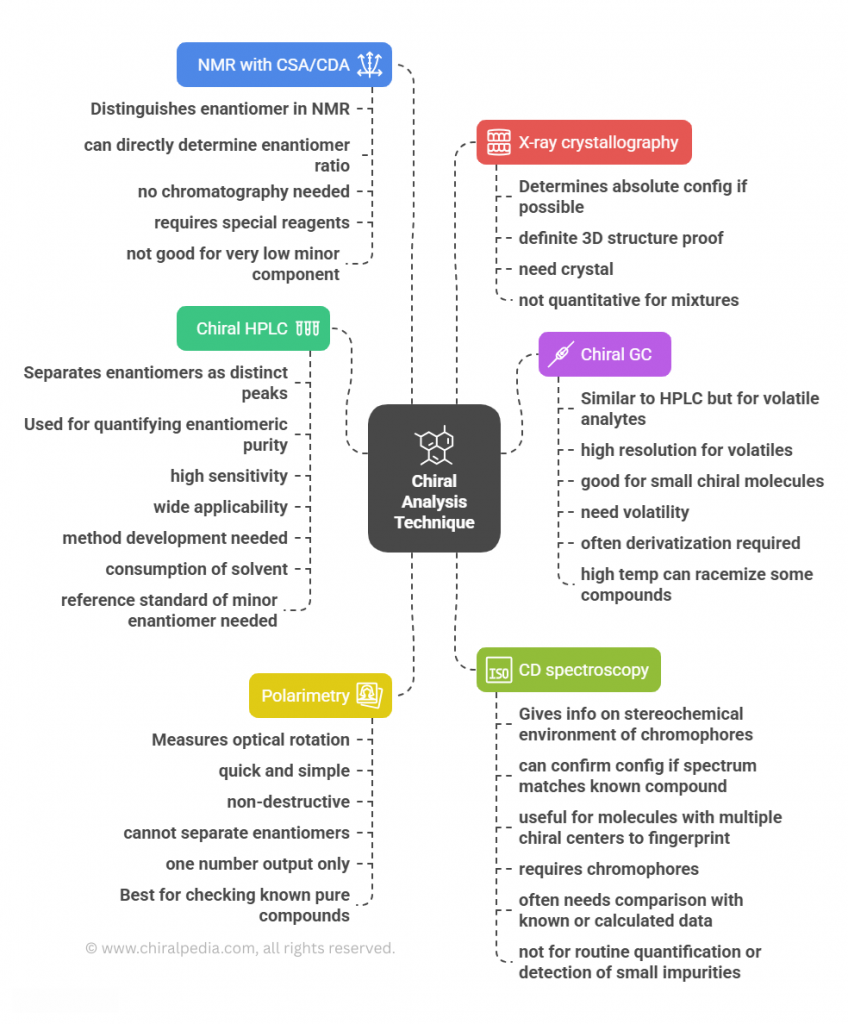
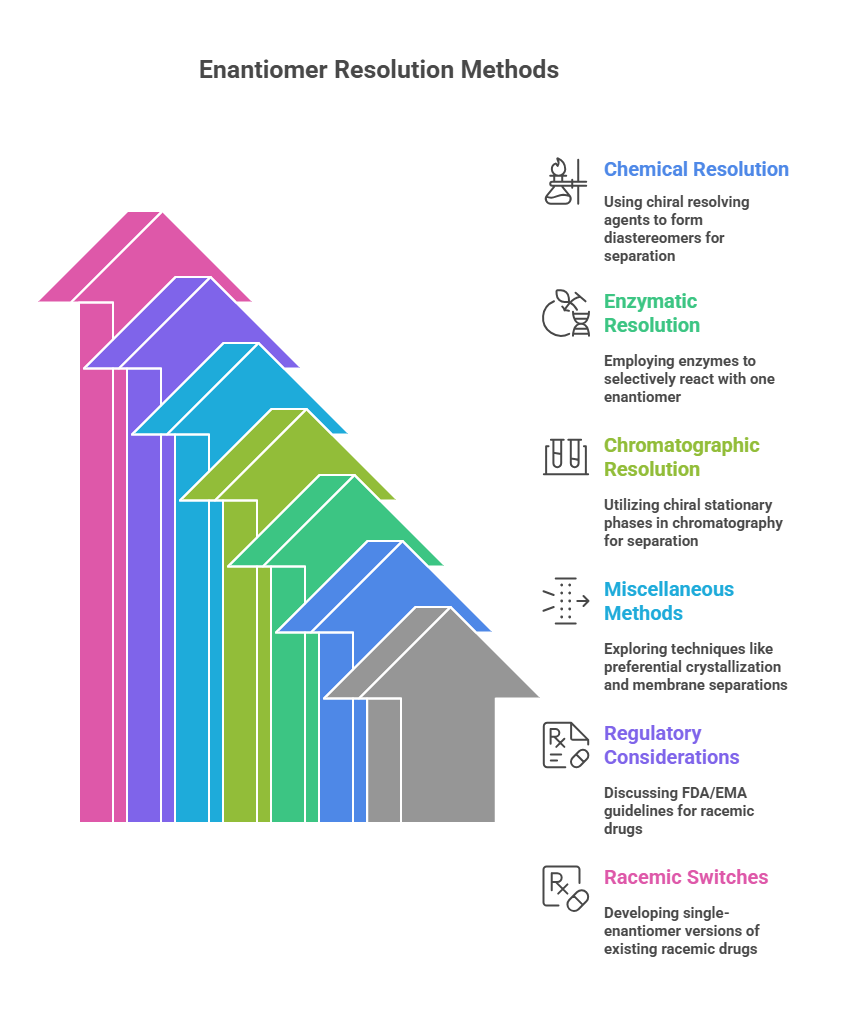
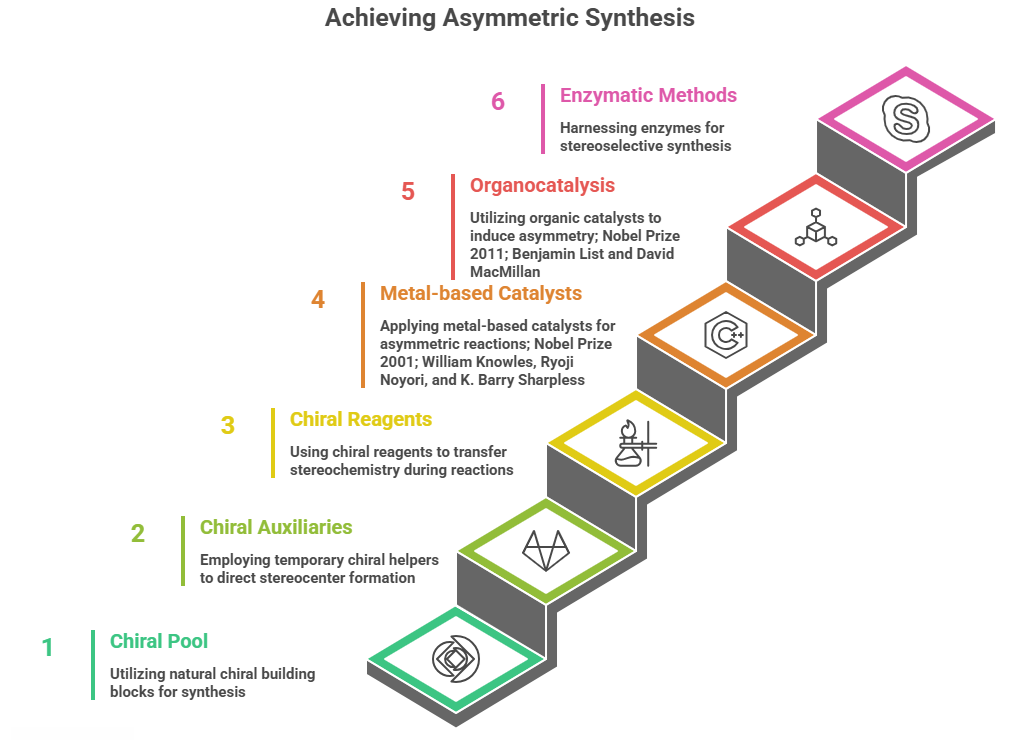
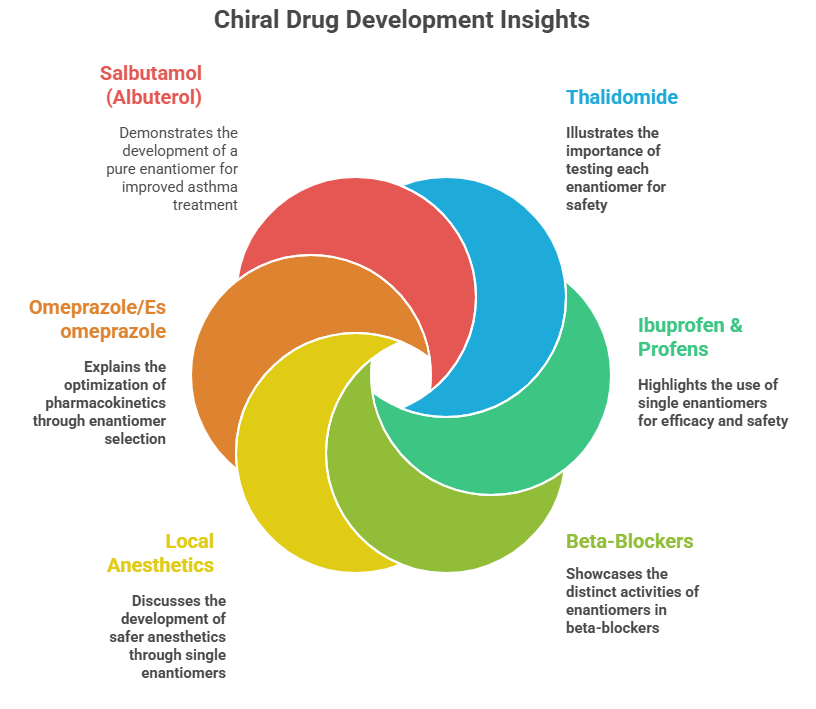
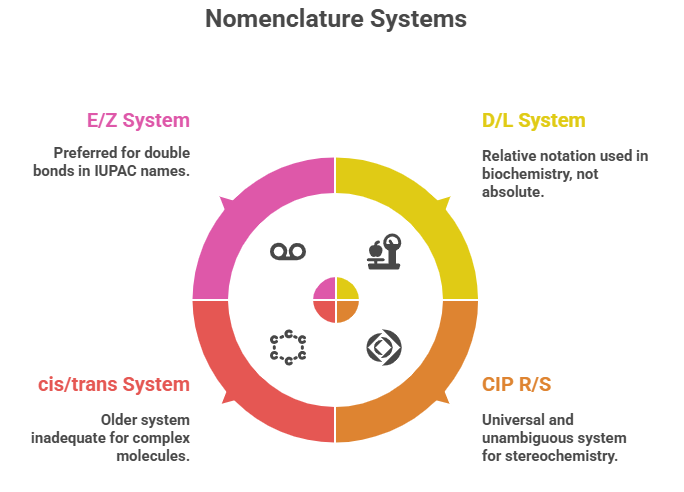
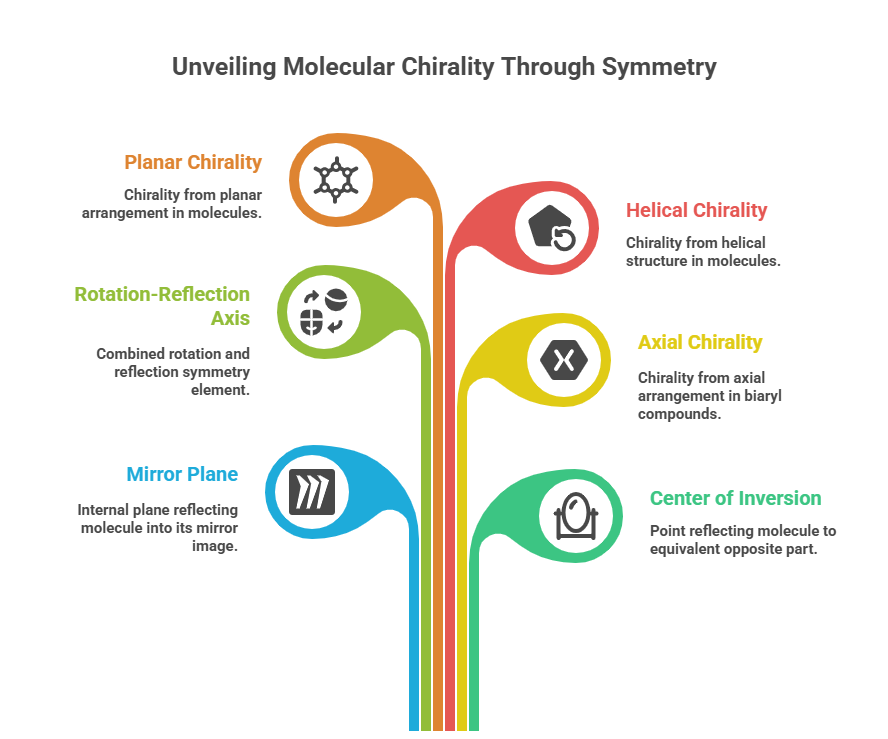
🧭 The Molecular Grammar of Medicines: Isomerism, Chirality, and Stereochemical Relationships Explained
“Where molecules speak, stereochemistry gives them meaning“ In pharmaceuticals, structure is language — and stereochemistry is its grammar. The way atoms arrange in 3D space shapes how drugs work, how they are regulated, and how safe they are. This Chiralpedia tutorial simplifies the molecular “grammar” behind drug behavior and innovation. When Structure Speaks Every drug molecule has a story written in its structure. Two compounds may share the same formula yet act entirely differently due …