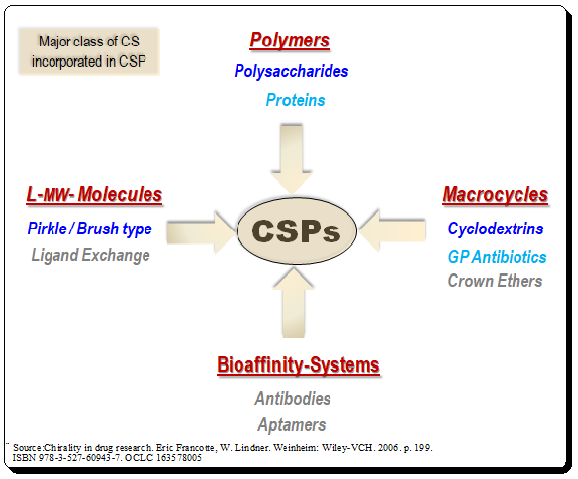
Lead
Proteins are complex, high-MW biopolymers composed of L-amino acids and possess ordered 3D-structure. They are known to bind /interact stereoselectively with small molecules reversibly, making them extremely versatile CSPs for chiral separation of drug molecules. Number of CSPs has been developed by immobilizing proteins. These type of CSPs operate under RP-mode; (phosphate buffer and organic modifiers)
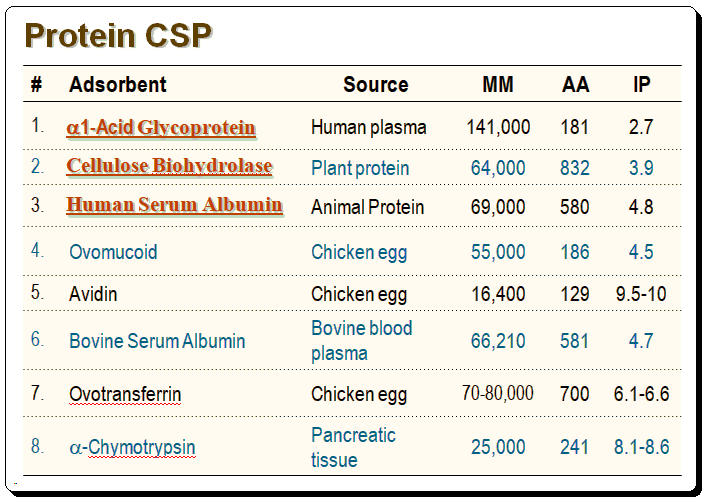
Characteristics of proteins used in commercial CSPs
Mechanism
Protein polymer remains in twisted form because of the different intramolecular bonding; these bonding are responsible for different type of chiral loops/grooves present in the protein molecule. Separation mechanism of proteins rely on unique combination of hydrophobic and polar interactions. H-bonding and charge transfer may also contribute to enantioselectivity by which the analytes are oriented to chiral surfaces. Mechanism of chiral recognition is not well understood
Strength
- Good efficiency
- Enantiomers can often be separated in an underivatized form with high enantioselectivity
Weakness
- Low loading capacity
- Protein phases are expensive
- Extremely fragile; Delicate to handle
- Very low Column efficiency
- Cannot invert elution order
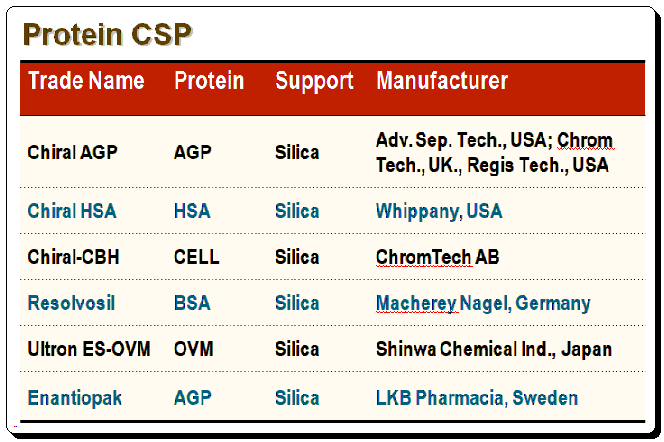
Commercially available protein-based CSPs
Application
The protein-based chiral columns have the distinct advantage that derivatization of racemates is rarely necessary for sufficient enantiomer resolution. Compounds that are neutral, basic, or acidic can typically be injected directly without needing to be treated first. According to reports in the literature, the OVM and AGP columns are probably the most adaptable immobilized proteins for separating racemates, particularly synthetic compounds of pharmaceutical and agricultural interest. The OVM column is a preferred starting point because it typically exhibits higher resolution, more operating parameter flexibility, and better long-term stability than the AGP column. For about 80% of the compounds, the OVM column performed better separations than AGP when a variety of neutral, basic, and acidic drugs were resolved. For more detailed discussion on the application readerships may consult surveys provided by the manufacturer of each protein-based CSPs.
Disclaimer
Due to the field’s quick development, there may have been some unintentional omissions or inadequate technical specifications. If any omissions or updates needing to be made are added, we would be pleased to include them. To draw attention to it, kindly write a comment. All technical specifications found here are reproduced as-is from the manufacturer’s literature and might have changed at the time of publication. All information given here is for furthering science and cannot be construed as professional advice.
Further reading
Lloyd R. Snyder, Joseph J. Kirkland, Joseph L. Glajch, Practical HPLC Method Development, pages. 537-615, 1997, John Wiley & Sons, Inc. ISBN 0-471-00703-X
Francotte, Eric; Lindner, Wolfgang (2006). Chirality in drug research. Eric Francotte, W. Lindner. Weinheim: Wiley-VCH. ISBN 978-3-527-60943-7. OCLC 163578005.
Narayanan SR. Immobilized proteins as chromatographic supports for chiral resolution. J Pharm Biomed Anal. 1992 Apr;10(4):251-62. DOI: 10.1016/0731-7085(92)80037-n. PMID: 1420455.
Bi C, Zheng X, Azaria S, Beeram S, Li Z, Hage DS. Chromatographic Studies of Protein-Based Chiral Separations. Separations. 2016 Sep;3(3):27. DOI:10.3390/separations3030027.
Carla Fernandes, Joana Teixeira, Madalena M. M. Pinto, and Maria Elizabeth Tiritan, Strategies for Preparation of Chiral Stationary Phases: Progress on Coating and Immobilization Methods, Molecules. 2021 Sep; 26(18): 5477.DOI: 10.3390/molecules26185477
Hassan Y. Aboul-Enein, Imran Ali, Chiral Separations by Liquid Chromatography And Related Technologies, 2003, Marel Dekker Inc. ISBN: 0-8247-4014-9
Teixeira, Joana; Tiritan, Maria Elizabeth; Pinto, Madalena M. M.; Fernandes, Carla (2019-02-28). “Chiral Stationary Phases for Liquid Chromatography: Recent Developments”. Molecules. 24 (5): 865. doi:10.3390/molecules24050865. ISSN 1420-3049. PMC 6429359. PMID 30823495.
Chiral analysis. Wikipedia, Wikipedia Foundation, 28/12/2022. https://en.wikipedia.org/wiki/Chiral_analysis