Lead
Many chromatographers develop HPLC separations on a daily basis. The philosophy behind method development is founded on a number of factors. Today, chromatographic separation and how it varies depending on the sample and experimental conditions have a good practical understanding. This understanding of the chromatographic process should serve as the foundation for any methodological approach to HPLC method development. Most of the time, a desired separation can be easily accomplished with a minimal number of experiments. In other situations, extensive experimentation may be required. A sound method-development strategy should only call for the number of experimental runs required to produce the desired outcome.
Challenges
Even for a skilled liquid chromatographer, developing a reliable, robust HPLC method can be challenging. Problems with liquid chromatography (LC) typically fall into one of two categories. Instrumentation is a problem in the first category. It can be annoying and time-consuming to isolate issues with check valves, mixers, detector lamps, and other hardware, but once the source has been found, the fix is frequently straightforward. Consistent preventive maintenance can help you avoid many instrument issues. The second kind of issue is related to the actual separation. Drifting retention, insufficient separation, and poor peak shape are a few examples of separation issues. Unfortunately, the signs of separation issues can be easy to spot, but pinpointing the root of the issue can be challenging, and fixing it may seem impossible. The separation issues mostly can be resolved through a systematic method development (MD) strategy. Besides sample preparation developing an HPLC method involves four basic steps: scouting, optimization, robustness testing, and validation. Scouting involves screening various column and eluent conditions.
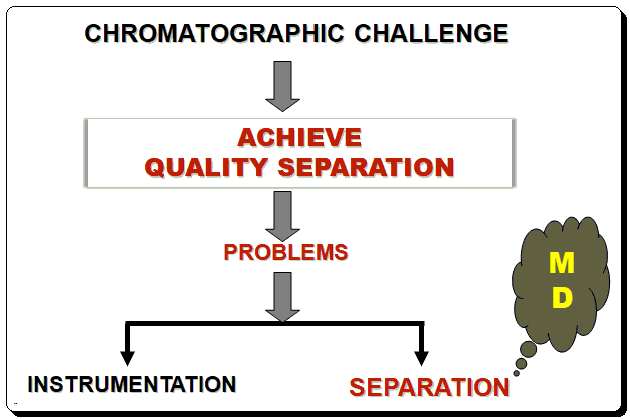
Starting out with a good method is one of the most important aspects of minimizing separation issues. A good method makes it much simpler to maintain operation within parameters and address issues as they arise. When developing a method, one should aim for good separation, adequate analysis time, and a manageable backpressure.
Getting started
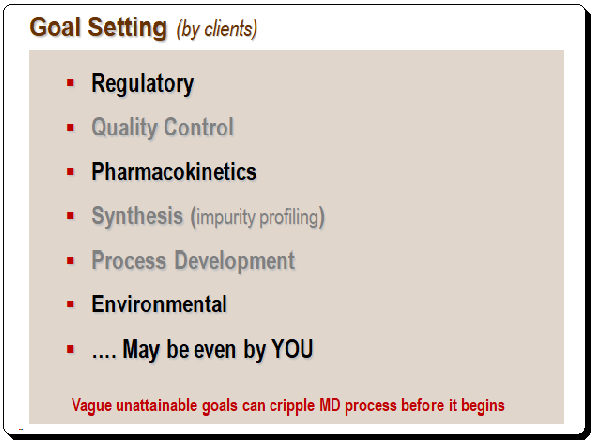
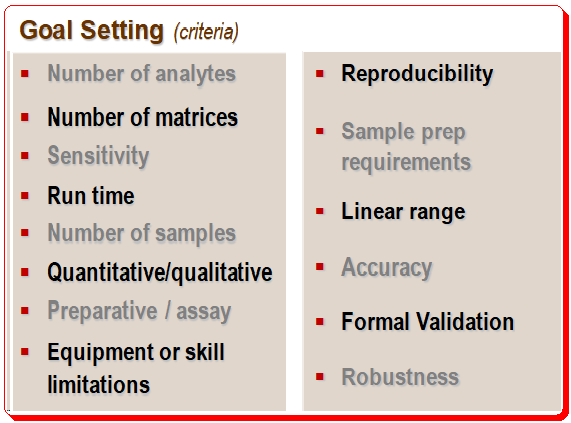
It is important to define the objectives of HPLC separation clearly, particularly the use and context. The client and the criteria being considered both define the goal. The illustration below provides an example of goal setting.
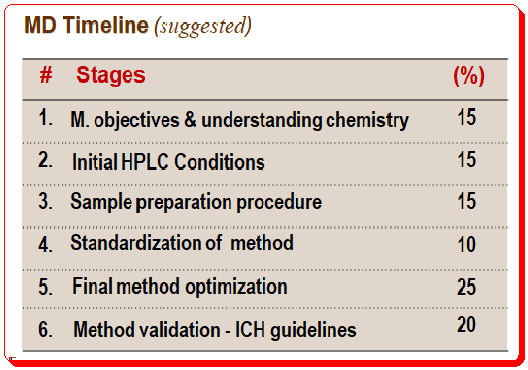
Method development timeline
If you start developing a method without proper planning, you may get an adequate separation but the likelihood of a robust method that is tolerant of small changes is less than if you had used a systematic approach. A typical steps in method development and the time line is given below.
Chromatographic separation process
Any methodical approach to developing an HPLC method should be based on an understanding of the chromatographic procedure. The illustration below shows the separation process schematically.
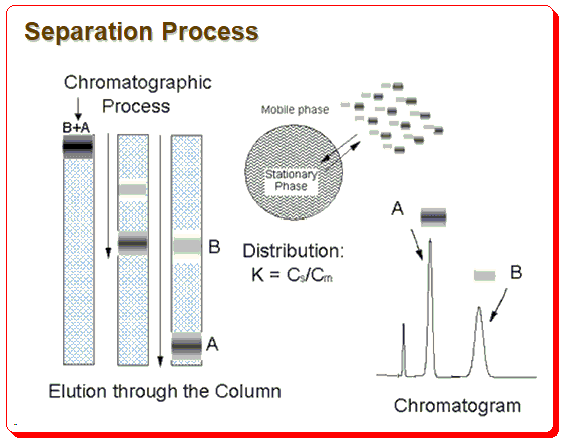
The difference in analyte distribution between the stationary and mobile phases serves as the basis for the separation. The chromatographer must carefully select and use the necessary tools, such as the column, mobile phase composition, pH, etc., to control the distribution of the analyte between the stationary phase and mobile phase in order for chromatographic separation to be successful.
Separation Process model: inputs (variables) and output (responses)
Experimenter’s first objective is to understand the separation process in terms of the Controlled inputs (variables), uncontrolled variables, and output (responses). . Mathematically, the response, or separation of analytes in this case, can be defined as a function of controlled inputs. The typical process model is shown below. Given below is a typical process model.
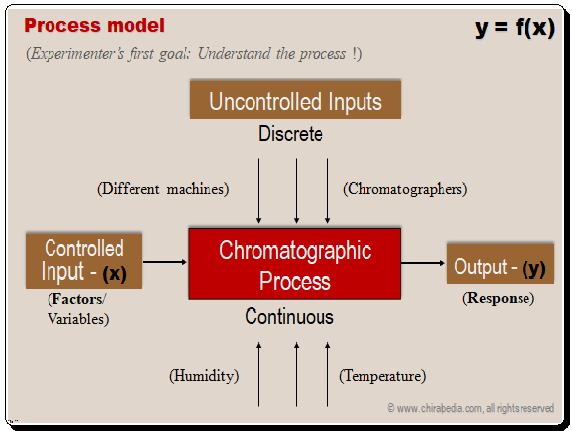
The most important controlled variables/factors include mobile phase composition, flow rate, pH, buffer concentration, pH, temperature, stationary phase, and, etc. Chromatographers, machines, humidity, temperature, and other uncontrolled inputs are a few examples. The responses include resolution, run time, capacity factor, tailing, and chromatographic optimization function.
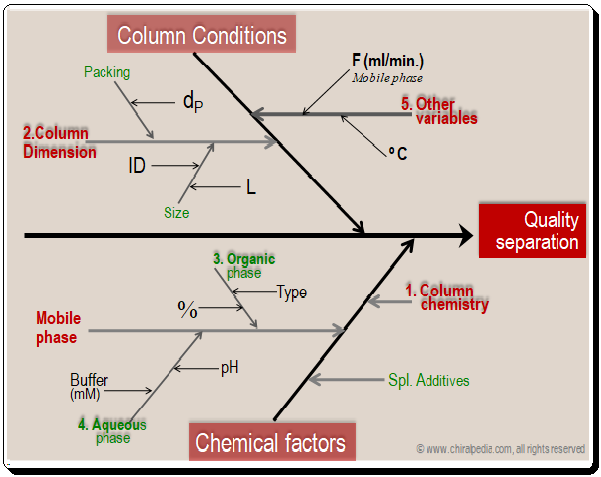
Factors influencing separation: Cause and effect diagram
Let’s look at the cause and effect diagram to better understand how to manage the variables/factors to achieve quality separation. Detailed analysis of these factors and how they influence resolution will be discussed in the forthcoming blogs.
This multipart series on HPLC method development as indicated below is an effort to narrate how to put your best foot forward and increase your chances of a successful HPLC separation.
#HPLC_method_development
Part 1: Begin right – Selecting tools
Part 2: Tools to measure quality of separation
Part 3: Role of solvent in controlling selectivity
Part 4: Varying column conditions to improve resolution
Part 5: Other variables to achieve desired resolution
For a detailed treatment of any of the above concepts consult books/articles suggested in the “Further reading” section.
Disclaimer
Further reading
Due to the field’s quick development, there may have been some unintentional omissions or inadequate technical specifications. If any omissions or updates needing to be made are added, we would be pleased to include them. To draw attention to it, kindly write a comment. All technical specifications found here are reproduced as-is from the manufacturer’s literature and might have changed at the time of publication. All information given here is for furthering science and cannot be construed as professional advice.
Lloyd R. Snyder, Joseph J. Kirkland, Joseph L. Glajch, Practical HPLC Method Development, pages. 600-615, 1997, John Wiley & Sons, Inc. ISBN 0-471-00703-X
John W. Dolan and Lloyd R. Snyder, Troubleshooting LC systems: A comprehensive approach to troubleshooting LC equipment and separations, 1989, Humana Press, Inc., New Jersey. ISBN 0-89603-151-9.
Montgomery, D. C. Design and Analysis of Experiments, 2019, John Wiley and Sons, New York. ISBN: 978-1-119-49244-3
Henrik Rasmussen (Editor), Satinder Ahuja (Editor), HPLC Method Development for Pharmaceuticals: Volume 8 (Separation Science and Technology), 2007, Academic Press Inc, ISBN-13 : 978-3527331291

The basis of HPLC method development is stated precisely, it helps the researchers or students who wants to work on HPLC. Thank you sir
Dear Dr.Selvakumar,
Thank you find it useful.
Dear Prof.
Thanks for starting the HPLC Method Development series; I’m looking forward to it because this subject is so widely applicable and relevant but given scant attention. Please keep them coming.
Dear Dr.Chandramouli,
Thanks for the encouraging words. Sure, you can look forward to the series on HPLC MD.