Lead
William H Pirkle developed these CSPs and are based on the ionic or covalent attachment of one enantiomer of an amino acid derivative to aminopropyl silica. Pirkle phases contain π-electron donor or acceptor rings substituted with H-bonding moieties. Donor–acceptor-type CSPs capitalize on synthetic or semi-synthetic chiral low-molecular-weight chiral selectors capable of recognizing chiral analytes by complementary assembly of nonionic attractive interactions.
Basic design and types
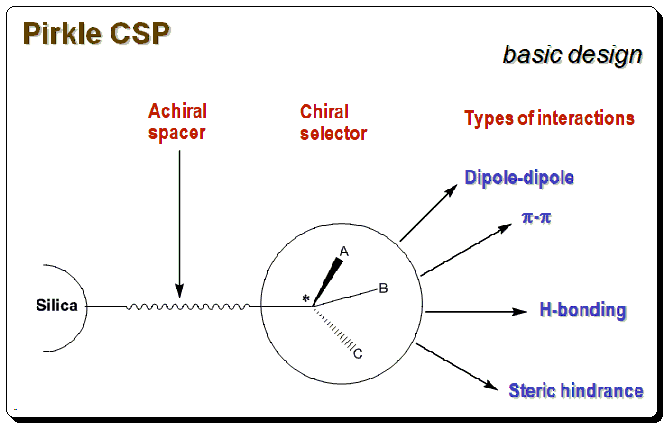
Generally speaking a chiral phase of the Pirkle type that combines structure-based selectivity with hydrogen bonding, π-π interactions, and dipole moments to increase selectivity. Bonded to high purity silica, fully endcapped. As a result of the covalent connection, it is compatible with all aqueous systems, as well as mobile phases, were employed. With only a brief period of equilibration between the normal phase and reversed phase , this column can be operated in both the two modes .
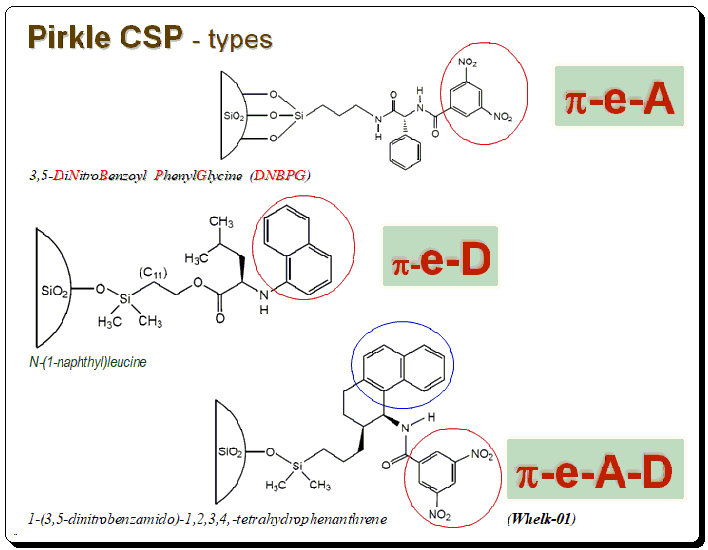
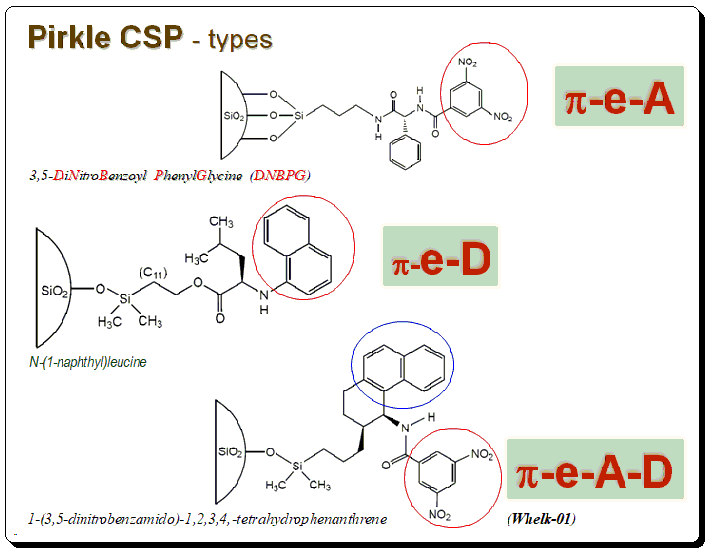
Pirkle’s CSPs falls into three types namely π-electron acceptor phases, π-electron acceptor phases and π-electron acceptor-donor phases. DNPBG, contains N(3,5-dinitrobenzoyl)-phenylglycine/phenyleucine charge transferring aromatic moieties. This is an acidic CSP capable of separating π-donor aromatic compounds. Napthylleucine phase is based on N-(1-naphthyl)leucine, covalently bonded to 5 mm silica through an ester linkage. Resolves DNB derivatives of amino acids as free acids when used in RP mode. Whelk-01 is the most revolutionary addition to Pirkle-Concept series is π-electron A/D phase. This concept is an innovative incorporation of both π-A/D characteristics, resulting a phase that can be used for a variety of compound.
Mechanism
Pirkle used the following justifications as the foundation for the creation of the Pirkle Chiral Stationary Phase: A chiral molecule must have a minimum of three points of interaction, at least one of which must be stereochemically dependent, in order to have different affinities for enantiomers. Chiral recognition takes place at binding sites with Pirkle CSPs. π-basic or acidic aromatic rings, acidic sites, basic sites, and steric interaction sites are the different categories of major binding sites. Aromatic rings have the potential to interact negatively. Hydrogen is provided by acidic sites for potential intermolecular hydrogen bonds. Also capable of forming hydrogen bonds are basic sites like electrons. Large groups may also engage in steric interactions.
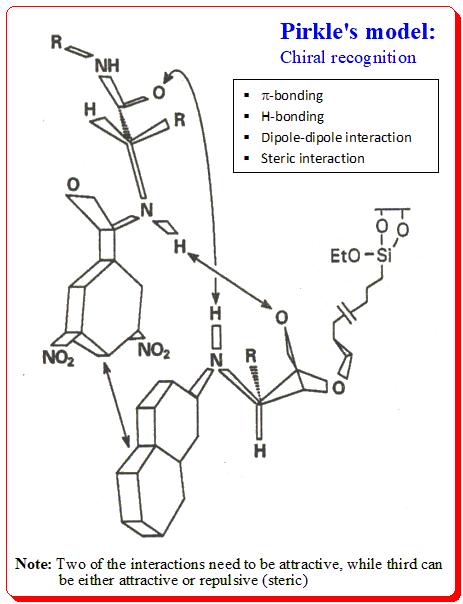
- Chiral separation is based on preferential binding of one enantiomer to the CSP (diastereomeric complex)
- π-D/A pair will form a charge transfer (CT) complex, when a charge can be transferred from the D to A molecule
- Success of this technique was interpreted by Pirkle, in the frame work of Dalgleish’s 3-point interaction
Application
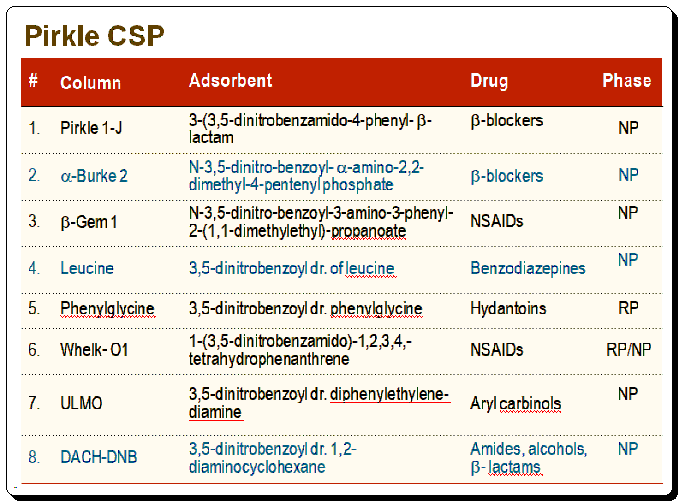
Strength
- Robust and allows high sample loads
- Separation of enantiomers of a wide variety of compound groups
- Column durability due to covalent phase bonding
- Ability to invert elution order
- Excellent chromatographic efficiency
- Available in analytical and Preparative size columns
- Accurate determination of ee, especially in trace analysis
- Universal solvent compatibility
Weakness
- Enantioselectivity and stability of Pirkle phase are generally much greater in NP
- DNB type system requires derivatives of the drug before chromatography to incorporate FGs & provide necessary interaction sites
Disclaimer
Due to the field’s quick development, there may have been some unintentional omissions or inadequate technical specifications. If any omissions or updates needing to be made are added, we would be pleased to include them. To draw attention to it, kindly write a comment. All technical specifications found here are reproduced as-is from the manufacturer’s literature and might have changed at the time of publication. All information given here is for furthering science and cannot be construed as professional advice.
Further reading
Francotte, Eric; Lindner, Wolfgang (2006). Chirality in drug research. Eric Francotte, W. Lindner. Weinheim: Wiley-VCH. ISBN 978-3-527-60943-7. OCLC 163578005.
Thomas E. Beesley, Review of Chiral Stationary Phase Development and Chiral Applications, LCGC Europe, 05-01-2011, Volume 24, Issue 5, Pages: 270–276
https://www.bgb-info.com/files/master/Regis/Regis_Catalog.pdf
https://www.registech.com/whelk-01/
Teixeira, Joana; Tiritan, Maria Elizabeth; Pinto, Madalena M. M.; Fernandes, Carla (2019-02-28). “Chiral Stationary Phases for Liquid Chromatography: Recent Developments”. Molecules. 24 (5): 865. doi:10.3390/molecules24050865. ISSN 1420-3049. PMC 6429359. PMID 30823495.
Christopher J. Welch, Reflections on Chirality in the Pharmaceutical Industry: Past, Present and Future, CHIRAL INDIA, 2016, November, Mumbai. India.
Chiral analysis. Wikipedia, Wikipedia Foundation, 17/11/2022. https://en.wikipedia.org/wiki/Chiral_analysis