Introduction
Chirality, or molecular handedness, plays a pivotal role in the chemistry of taste and smell. From the refreshing scent of mint to the sweet taste of artificial sweeteners, the chirality of molecules influences our sensory experiences in profound ways. In this blog, we will delve into the fascinating world of chiral compounds, exploring how they interact with our sensory receptors and shape the flavors and fragrances we encounter daily. By understanding the chemistry behind taste and smell, we can appreciate the intricate ways in which chirality affects our sensory perceptions.
Chirality in Flavour and Fragrance Compounds
Definition and Explanation
Chirality refers to a property of asymmetry where a molecule cannot be superimposed on its mirror image, much like how our left and right hands are mirror images but not identical. These non-superimposable mirror images are known as enantiomers. Chiral chemicals play a key role in the production of flavors and fragrances, influencing their aroma and taste. Enantiomers can exhibit distinct olfactory and gustatory profiles, making chirality a crucial factor in the chemistry of smell and taste.
Role of Enantiomers in Flavors and Scents
Enantiomers often interact differently with biological systems, leading to distinct flavors and scents. This phenomenon is well-illustrated by the chiral molecule limonene and caravone. . Both of them despite having the same molecular formula, their enantiomers produce entirely different olfactory experiences due to their chirality.
Examples of Chiral Compounds
Limonene: Limonene is a common chiral cyclic monoterpene found in citrus fruits. The R-enantiomer of limonene emits a sweet, orange-like aroma, whereas the S-enantiomer has a sharp, lemon-like scent. This stark difference in smell highlights the significant impact chirality can have on our sensory perceptions.

Limonene mirror-image twins and biological activity
The image illustrates two fruits, an orange and a lemon, along with their respective chemical structures. The chemical structure on the right top corresponds to (R)-(+)-limonene, a chiral molecule commonly found in oranges, while the structure on the right represents (S)-(-)-limonene, a chiral compound typically found in lemons. The chirality of these molecules plays a crucial role in their distinct aromas and flavors.
How Chiral Molecules Interact with Sensory Receptors
Overview of the Human Sensory System
The human sensory systems for taste and smell rely on specialized receptors that detect and differentiate a wide array of chemical compounds. These receptors are highly sensitive to the three-dimensional shapes of molecules, including their chirality. The interaction between chiral molecules and sensory receptors is fundamental to our perception of flavors and scents.
Interaction with Olfactory Receptors
Olfactory receptors are proteins located in the nasal epithelium that bind to odorant molecules and initiate a signal transduction pathway, leading to the perception of smell. Chiral molecules can bind to these receptors in different ways depending on their orientation. Odorant binding proteins are found in the mucus of the mammalian nose and also in the antennae of insects where they function as carriers of small molecules to and from the olfactory receptors. They are extremely stable and capable of binding a variety of different molecules.
Carvone is another excellent example of how chirality influences scent. The S-enantiomer of carvone smells like caraway seeds while the R-enantiomer smells like spearmint. These distinct aromas arise from the way each enantiomer interacts with olfactory receptors in our noses.
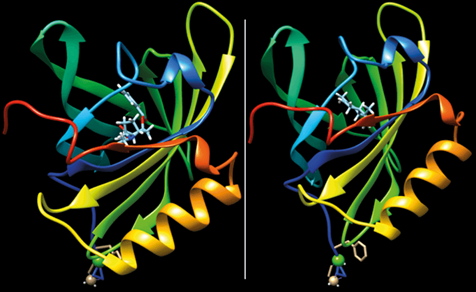
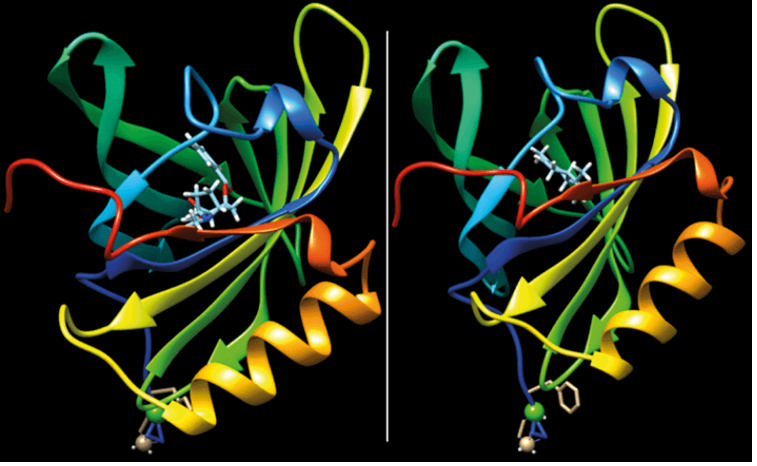
How Chirality Influences Smell
The interaction of chiral molecule carvone with the binding pocket of an artificial protein derived from porcine odorant binding protein. Chirality significantly influences smell because the two enantiomers, (R)-(-)-carvone (left) and (S)-(+)-carvone (right), fit differently into the protein’s binding site. This distinct fit alters the protein’s response, leading to different olfactory perceptions. The (R)-(-)-carvone is commonly associated with a caraway scent, while the (S)-(+)-carvone is linked to a spearmint scent. Thus, chirality is crucial in determining the specific aroma detected, as each enantiomer’s unique three-dimensional structure interacts differently with olfactory receptors.
Interaction with Taste Receptors
Taste receptors, located on the tongue, function similarly to olfactory receptors. They bind to tastant molecules and send signals to the brain, which interprets these signals as specific tastes. Chiral molecules can interact with taste receptors differently, leading to varied taste perceptions. For example, the sweet taste of aspartame is influenced by its chiral structure, which allows it to bind effectively to sweet taste receptors.
Examples of Chiral Substances Affecting Taste and Smell
Case Studies

Menthol: Menthol is a chiral compound found in peppermint. The l-enantiomer of menthol [(1 R ,2 S ,5 R )-2-isopropyl-5-methylcyclohexanol] produces a strong cooling sensation, while the d-enantiomer has a much weaker effect. This difference in sensory perception is due to the distinct interactions each enantiomer has with the cold receptors in our skin and mucous membranes.
Threonine: Threonine is a proteogenic amino acid with two chiral centers and hence exists in four stereoisomeric forms with the following configurations: (2S,3R), (2R,3S), (2S,3S) and (2R,3R). They are depicted below in Fischer projection formula.
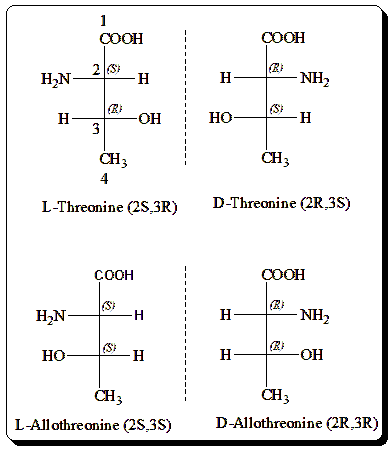
Impact of chirality in threonine’s chemistry
Chirality in threonine, with its two chiral centers, affects its 3D structure, influencing interactions with enzymes and receptors. Only the (2S,3R)-threonine is biologically active, fitting precisely into protein structures and metabolic pathways. This specific spatial arrangement is crucial for proper biochemical functioning, highlighting the importance of chirality in threonine’s chemistry.
L-Threonine is an essential amino acid, that is, humans must obtain it from foods because their bodies cannot synthesize it. Foods that contain high amounts of protein, such as meats, fish, eggs, and dairy products, are good sources of L-threonine. The isomers other than L-Threonine ( (D-, L–allo-, and D–allo-threonine) are rare in nature and perceived to have no value, nutritional or otherwise. This example illustrates how chirality can influence the taste of even fundamental biological molecules.
Aspartame: Aspartame is a widely used artificial sweetener composed of chiral amino acids. It has two stereogenic centers and exists in four stereoisomeric forms.
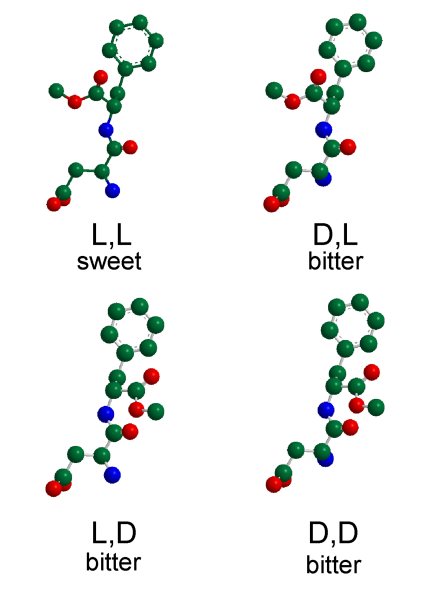
Source: Temussi, P. A. (2011). The good taste of peptides. Journal of Peptide Science, 18(2), 73–82. doi:10.1002/psc.1428
Impact of chirality on taste
The image depicts the structural variants of aspartame (molecular models of four aspartame stereoisomers), highlighting the impact of chirality on taste. Only the L,L-Aspartame with the configuration (S,S-) exhibits sweet taste, demonstrating how different chiral centers in molecules can drastically influence sensory perception and chemical properties.
Its sweet taste is a result of its chiral structure, which allows it to interact specifically with sweet taste receptors. The effectiveness of aspartame as a sweetener highlights the importance of chirality in taste modulation.
Impact on the Food and Fragrance Industry
The food and fragrance industries heavily rely on the unique properties of chiral compounds to create specific flavors and scents. Companies often isolate or synthesize enantiomerically pure compounds to ensure consistent and desirable sensory experiences. The stereochemistry of these compounds is crucial in product development, as the wrong enantiomer can lead to unwanted tastes or smells.
For example, the fragrance industry utilizes chiral terpenes and esters to formulate perfumes that are appealing and long-lasting. Similarly, the food industry employs chiral flavorings to enhance the taste of various products, from beverages to confectioneries. Understanding and controlling chirality is essential for producing high-quality consumer goods.
Conclusion
Chirality, the property of molecular handedness, plays a crucial role in shaping the chemistry of taste and smell. From the distinct aromas of limonene enantiomers to the varying tastes of amino acids, chirality influences our sensory experiences in profound ways. By understanding how chiral molecules interact with sensory receptors, we gain insight into the intricate mechanisms underlying taste and smell. This knowledge not only enhances our appreciation of the chemical complexity of our senses but also drives innovation in the food and fragrance industries. As research in this field continues to advance, we can expect new applications and discoveries that further highlight the importance of chirality in our sensory world.
Suggested Further Readings
https://www.scienceinschool.org/wp-content/uploads/2022/05/Issue58_Limonene.pdf
https://theanalyticalscientist.com/fields-applications/sniffing-out-chirality
https://www.linkedin.com/pulse/chiral-chemicals-market-harnessing-asymmetry-innovative-steve-jason
https://www.caltech.edu/about/news/new-tools-creating-mirrored-forms-molecules-84182
Nematollahi N, Ross PA, Hoffmann AA, Kolev SD, Steinemann A. Limonene Emissions: Do Different Types Have Different Biological Effects? Int J Environ Res Public Health. 2021 Oct 7;18(19):10505. doi: 10.3390/ijerph181910505.
Limonene in Citrus: A String of Unchecked Literature Citings? Lise Kvittingen, Birte Johanne Sjursnes, and Rudolf Schmid. J. Chem. Educ. 2021, 98, 11, 3600–3607. https://doi.org/10.1021/acs.jchemed.1c00363
https://www.britannica.com/science/menthol
http://www.leffingwell.com/Chem_Spec_Mag-1.pdf
https://www.linkedin.com/pulse/chiral-chemicals-market-harnessing-asymmetry-innovative-steve-jason
https://www.acs.org/molecule-of-the-week/archive/t/threonine.html
https://en.wikipedia.org/wiki/Threonine
Ronald Bentley, The Nose as a Stereochemist. Enantiomers and Odor, Chemical Reviews 2006 106 (9), 4099-4112. doi: 10.1021/cr050049t
https://www.3dchem.com/aspartame.asp#
Temussi PA. The good taste of peptides. J Pept Sci. 2012 Feb;18(2):73-82. doi: 10.1002/psc.1428. Epub 2011 Dec 7. PMID: 22147342.
Other Blogs on #ChiralityOnFriday
1. Introduction to Chirality: Understanding the Basics
2. Molecular Handedness: How Chirality Shapes Molecules
3. Chirality in Nature: From DNA to Snail Shells
4. The Chemistry of Taste and Smell: How Chirality Affects Senses
5. Chiral Chemistry in Everyday Life: Hidden Handedness Around Us
7. https://chiralpedia.com/blog/chirality-in-materials-science-designing-with-handedness/
8. Chirality in Space: Cosmic Asymmetry and the Origins of Life
9. The Future of Chirality: Innovations and Emerging Trends
10. Chirality and You: Understanding and Appreciating Molecular Handedness