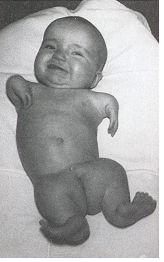
Thalidomide was a racemic therapeutic and prescribed to pregnant women to control nausea and vomiting. The drug was withdrawn from world market when it became evident that the use in pregnancy causes phocomelia (clinical conditions where babies are born with deformed hand and limbs). Later in late 1970s, the thalidomide enantiomers were separated and enantioselective studies indicated that the (R)- enantiomer, the good partner, is an effective sedative, the (S)-enantiomer, the evil partner, harbors teratogenic effect and causes fetal abnormalities. The problem with thalidomide is one of the reasons that chiral separation and analysis has become so important in drug design and development chemistry. [Read more on chiral separation and analysis @ https://en.wikipedia.org/wiki/Chiral_analysis].
Events of thalidomide tragedy – an overview
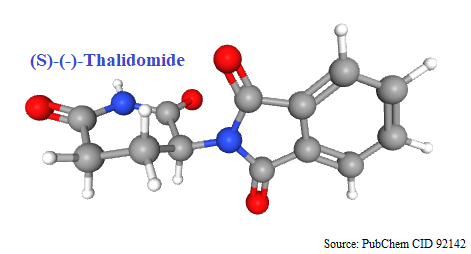
1956: Introduced in world market
1960: Teratogenic effect noticed
1961: Withdrawn from world market
1970: Resolved enantiomers
Chirality and drug toxicity
Thalidomide carries one stereogenic center at the C-3 of the 2,6-piperidine-2,6-dione ring and hence the molecule exists as an enantiomeric pair. The (R)-enantiomer has the desired sedative effect while the (S)-enantiomer harbors embryo-toxic and teratogenic effect.
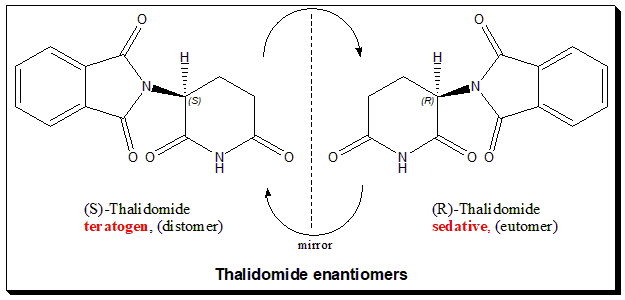
The hypothesis that the tragedy could be avoided in this case by using a single enantiomer is misleading and pointless, because it was later demonstrated that the “safe” R-thalidomide undergoes an in vivo chiral inversion to the “teratogenic” S-thalidomide. Under biological conditions, the enantiomers interconvert [bidirectional chiral inversion – indicated by curved arrows; (R)- to (S)- and vice versa]. Thalidomide tragedy (https://pubmed.ncbi.nlm.nih.gov/21507989/) led to profound and sustainable change in toxicity testing, regulatory control and the ways we look at chiral molecules. Today enantiomers are treated as two different chemical entities at least with respect to chiral drugs, like a polypharmacy.
Return of thalidomide
However, there is renewed interest in restricted use of thalidomide because of its immunomodulatory, anti-angiogenic, and anti-inflammatory effects.
Therapeutic category
Immunomodulatory agent
Nomenclature
(RS)-2-(2,6-dioxopiperidin-3-yl)-1H-isoindole-1,3(2H)-dione
Exercise
Learn the numbering and nomenclature of the molecule
Understand the terms employed – eutomer, distomer, phocomelia, teratogen
Will chiral switching of racemic thalidomide to (R)-thalidomide, the eutomer, work?
References
Tokunaga, E., Yamamoto, T., Ito, E. et al. Understanding the Thalidomide Chirality in Biological Processes by the Self-disproportionation of Enantiomers. Sci Rep 8, 17131 (2018). https://doi.org/10.1038/s41598-018-35457-6; https://rdcu.be/cVeXt
Chiral drugs. Wikipedia, Wikipedia Foundation, 18/08/2022. https://en.wikipedia.org/wiki/Chiral_drugs and references therein
Chiral analysis. Wikipedia, Wikipedia Foundation, 20/08/2022. https://en.wikipedia.org/wiki/Chiral_analysis and references therein
Lien Ai Nguyen, Hua He, and Chuong Pham-Huy, Chiral drugs: An overview, Int J. Biomed. Sci. 1,2,85-100, 2006; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3614593/
Mellin, Gilbert W.; Katzenstein, Michael (1962-12-06). “The Saga of Thalidomide”. New England Journal of Medicine. 267 (23): 1184–1193.
De Camp, Wilson H. (1989). “Letter to the editor”. Chirality. 1 (2): 97–98.
James H Kim , Anthony R Scialli, Thalidomide: the tragedy of birth defects and the effective treatment of disease, Toxicol Sci. 2011 Jul;122(1):1-6. DOI: 10.1093/toxsci/kfr088
https://www.understandinganimalresearch.org.uk/news/sixty-years-on-the-history-of-the-thalidomide-tragedy
Hassan Y. Aboul-Enein, Irving W. Wainer, The Impact of Stereochemistry on Drug Development and Use, John Wiley & Sons, New York, 1997. https://www.wiley.com/en-
Harkishan Singh and V.K. Kapoor. Medicinal chemistry and pharmaceutical chemistry, Vallabh Prakashan, New Delhi, Page 689, 2012.
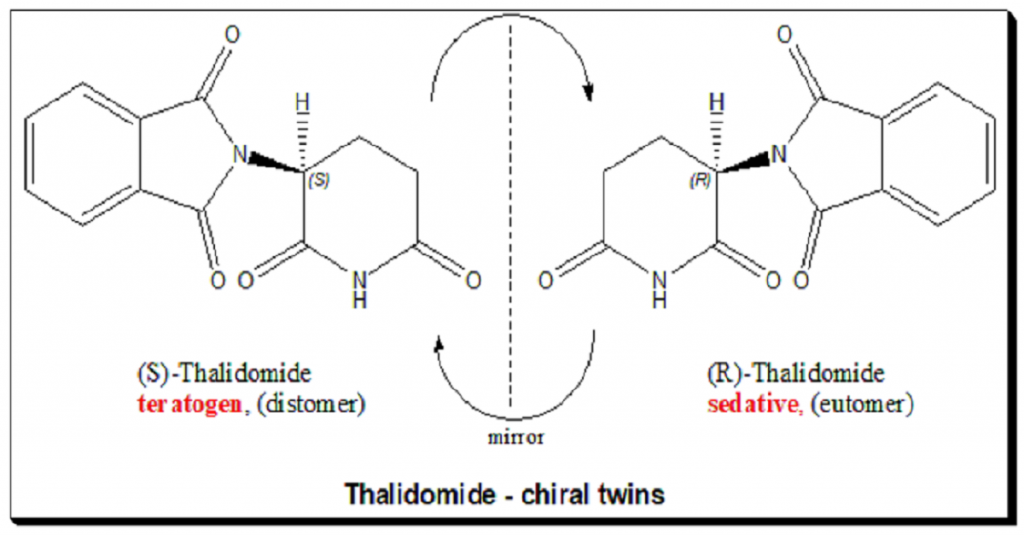

Well written and very useful article which tells about the role of Chirality.in toxicity testing during drug development .