Cis-trans and E-Z notation: choose your side
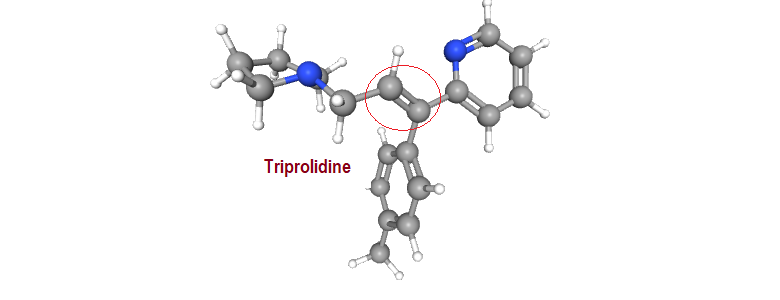
“Pharmacological studies confirm the high activity of triprolidine and the superiority of (E) over corresponding (Z) isomers as H, antagonist” ( Ref: – – – from “Wilson and Gisvold’s Textbook of organic medicinal and pharmaceutical chemistry, 2010”). “Triprolidine is 2-[(E)-1-(4-methylphenyl)-3-pyrrolidin-1-ylprop-1-enyl]pyridine”, the IUPAC name. To understand the above statements one need to be familiar with the “cis-trans and E-/Z- nomenclature. How to translate the name to structure and vice versa? This blog is basically to discuss …