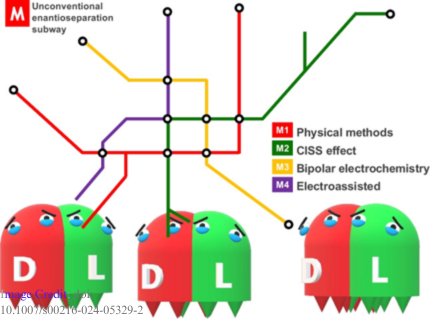
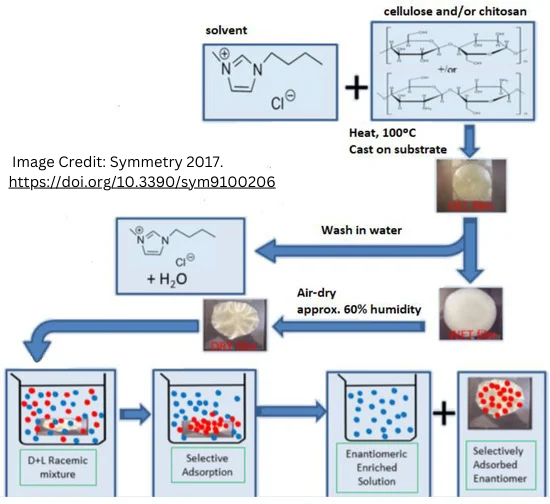
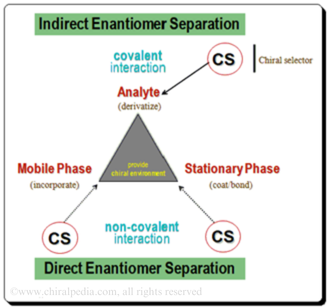
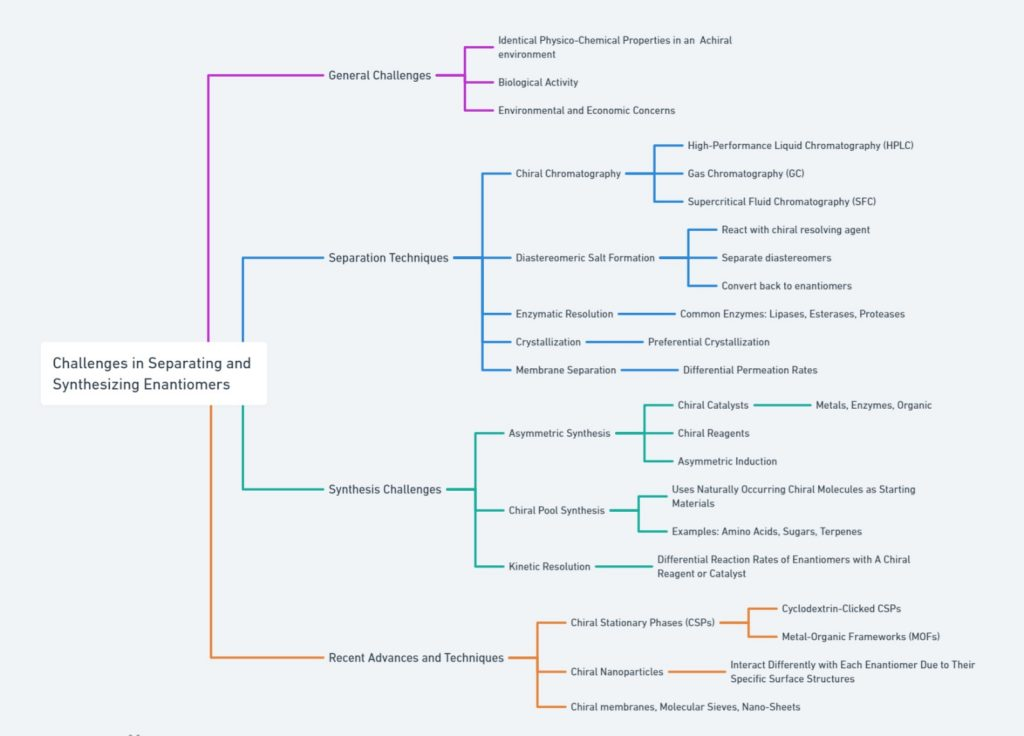
P3. Chirality in Medicine: From Discovery to Disaster
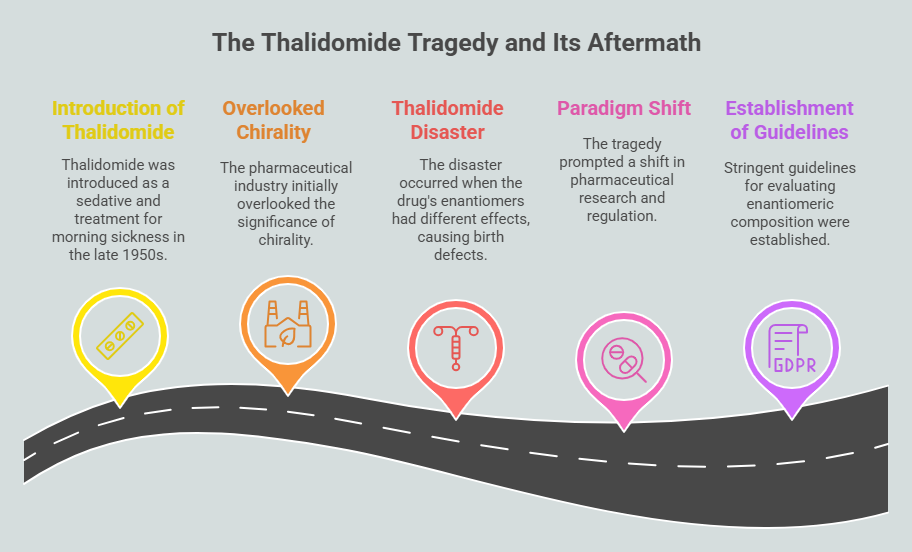
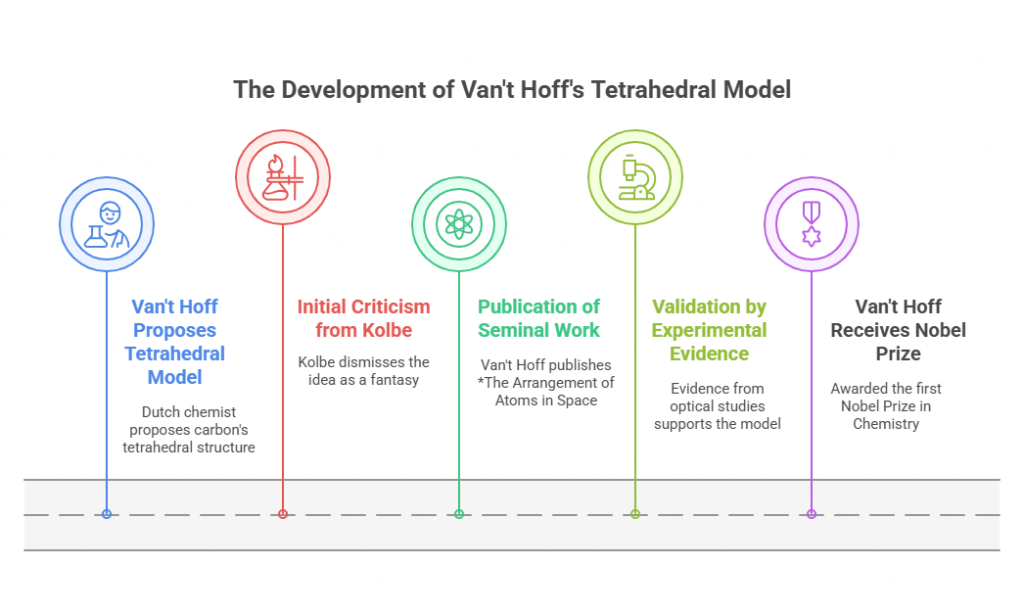
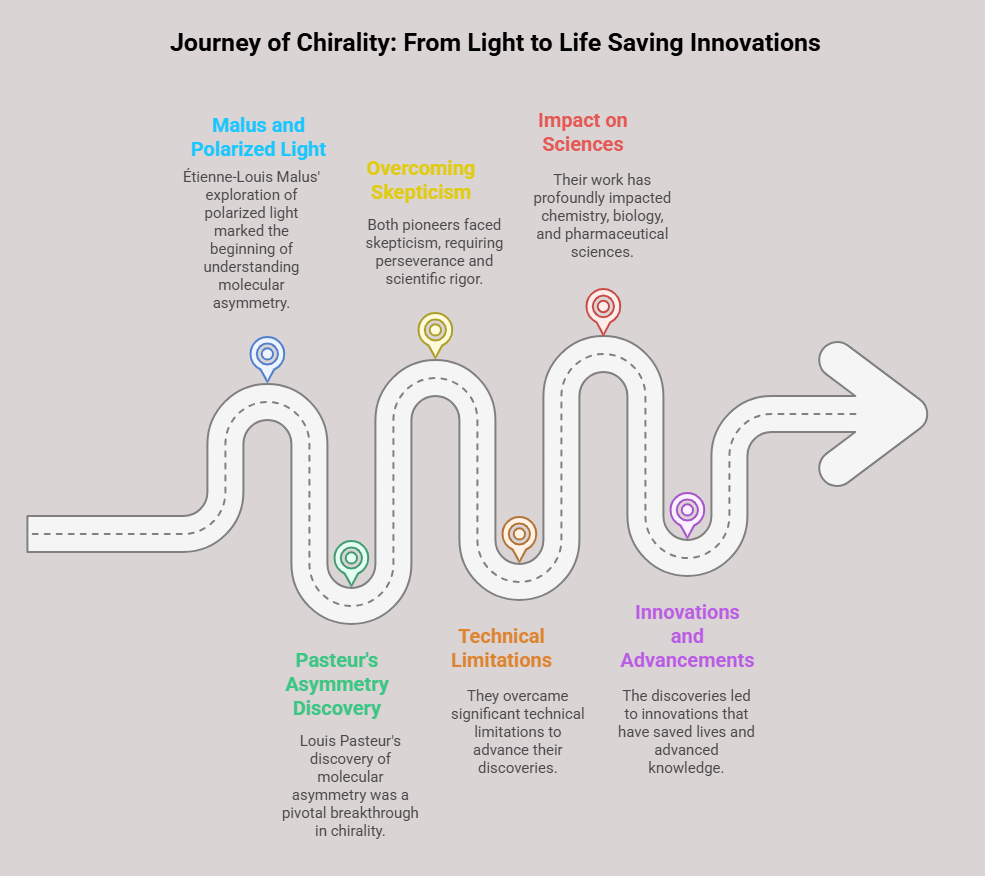
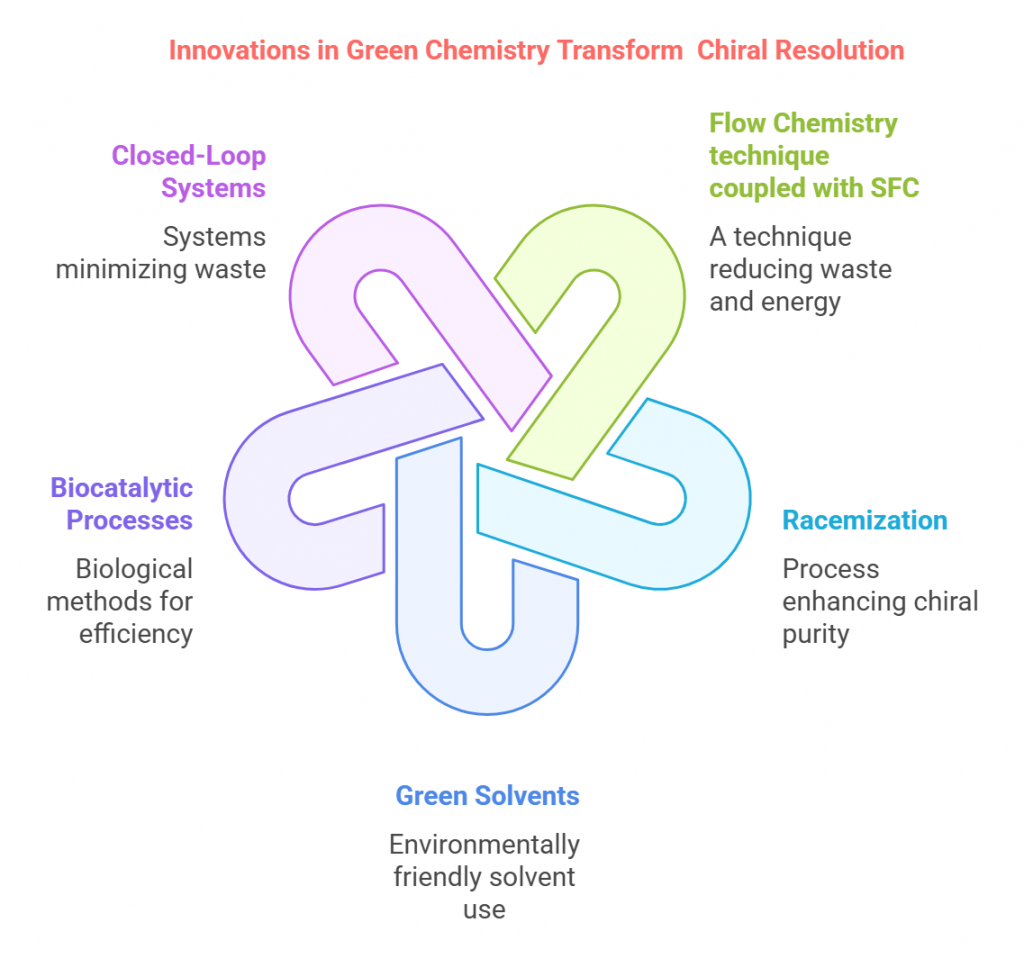
The intersection of chirality and medicine is a compelling narrative that reveals both the promise and peril of molecular asymmetry. From its earliest applications in pharmacology to the tragic consequences of overlooking enantiomeric differences, the role of chirality in medicine underscores the profound impact of stereochemistry on human health. This chapter delves into the breakthroughs, challenges, and lessons learned as chirality became a cornerstone of drug development. Early Discoveries: The Physiological Impact of Chirality The …
P3. Chirality in Medicine: From Discovery to Disaster Read More »