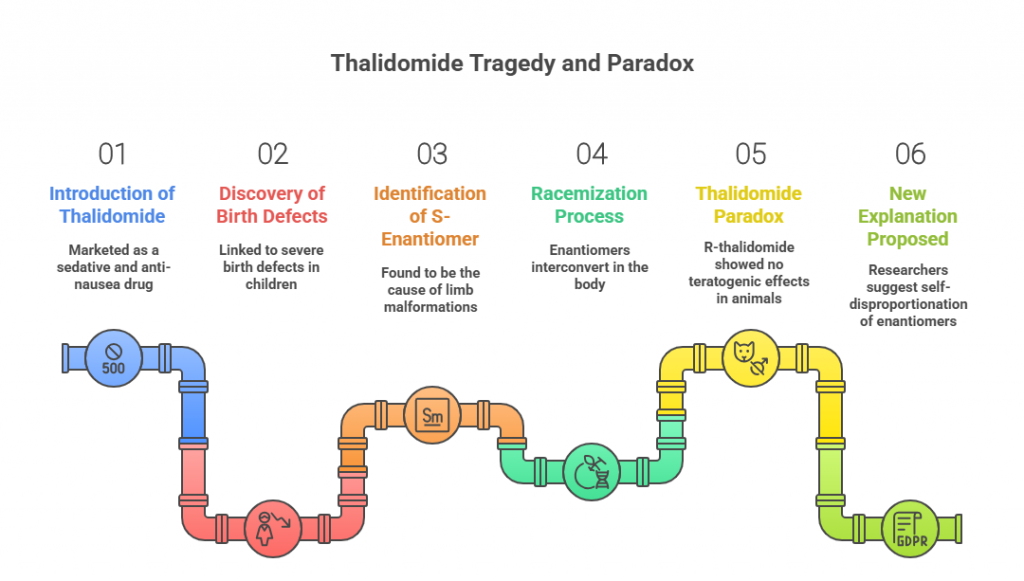
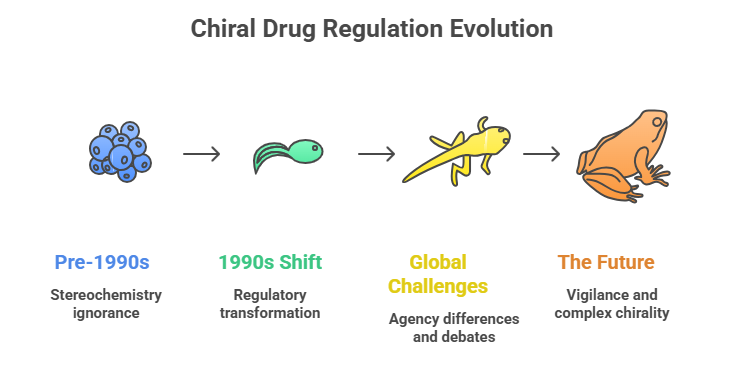
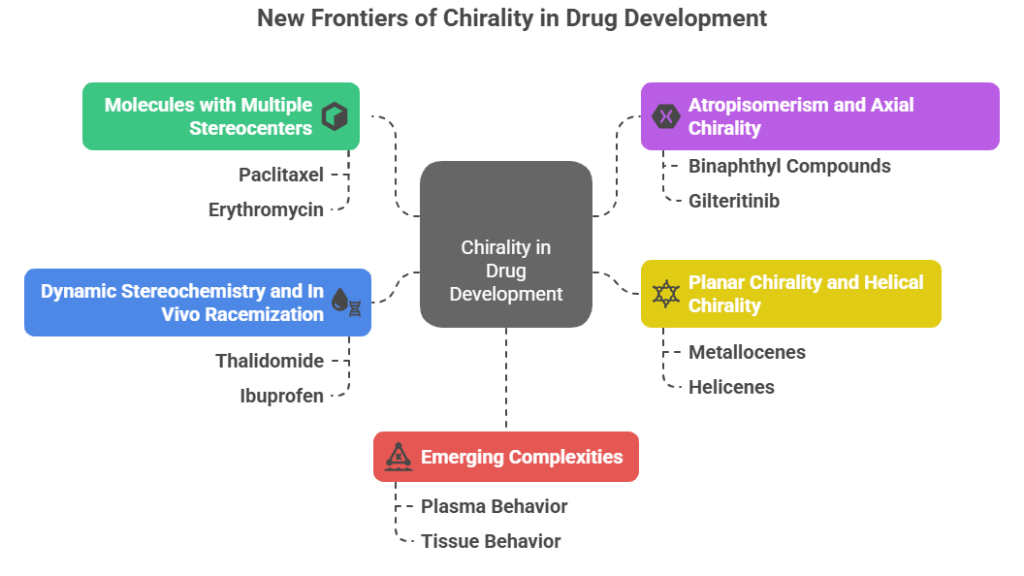
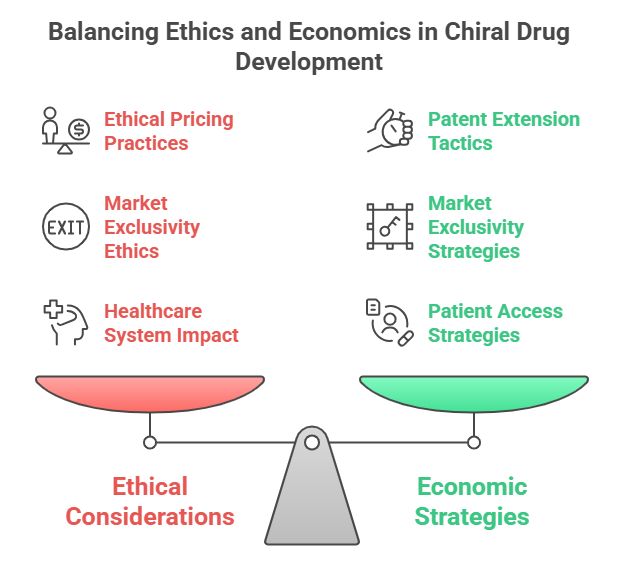
Part 3: Nomenclature and Configuration
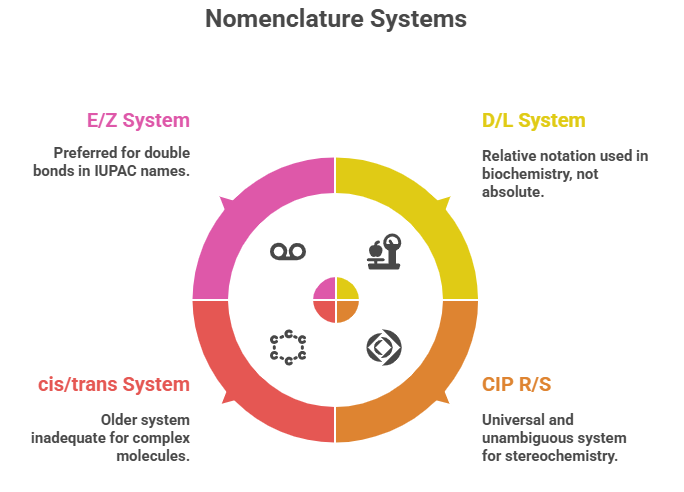
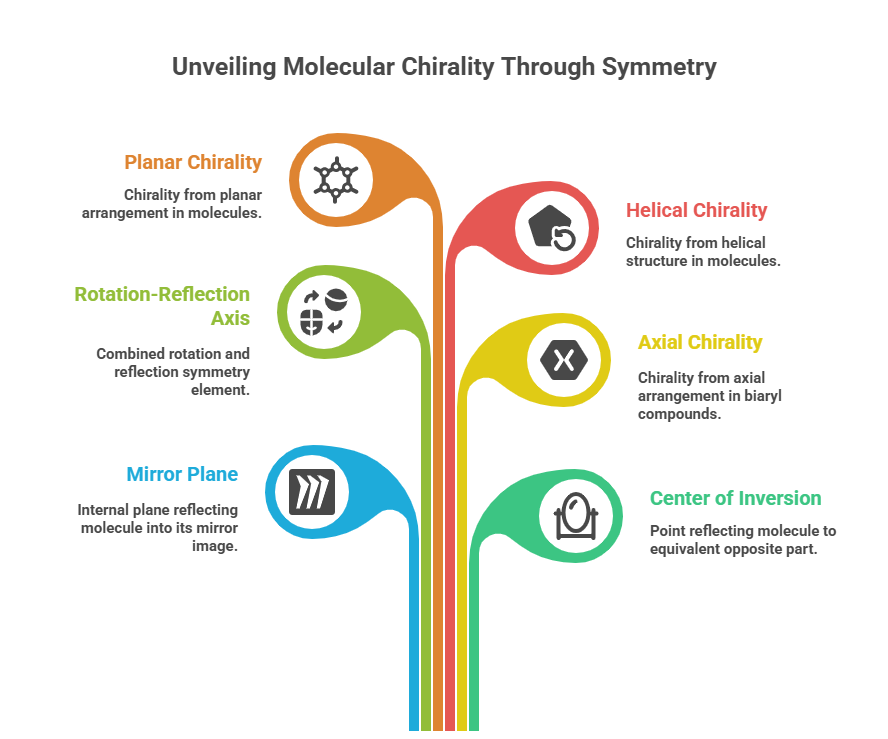
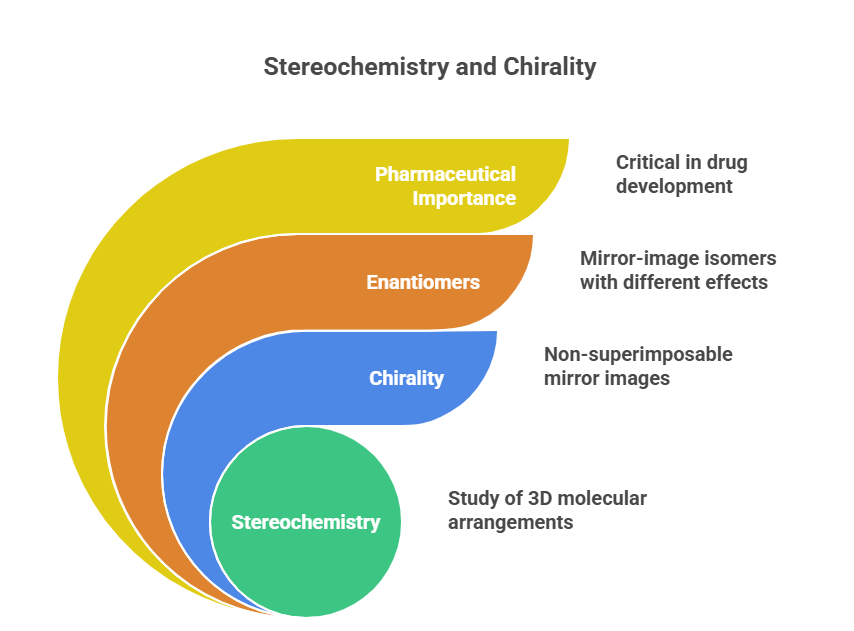
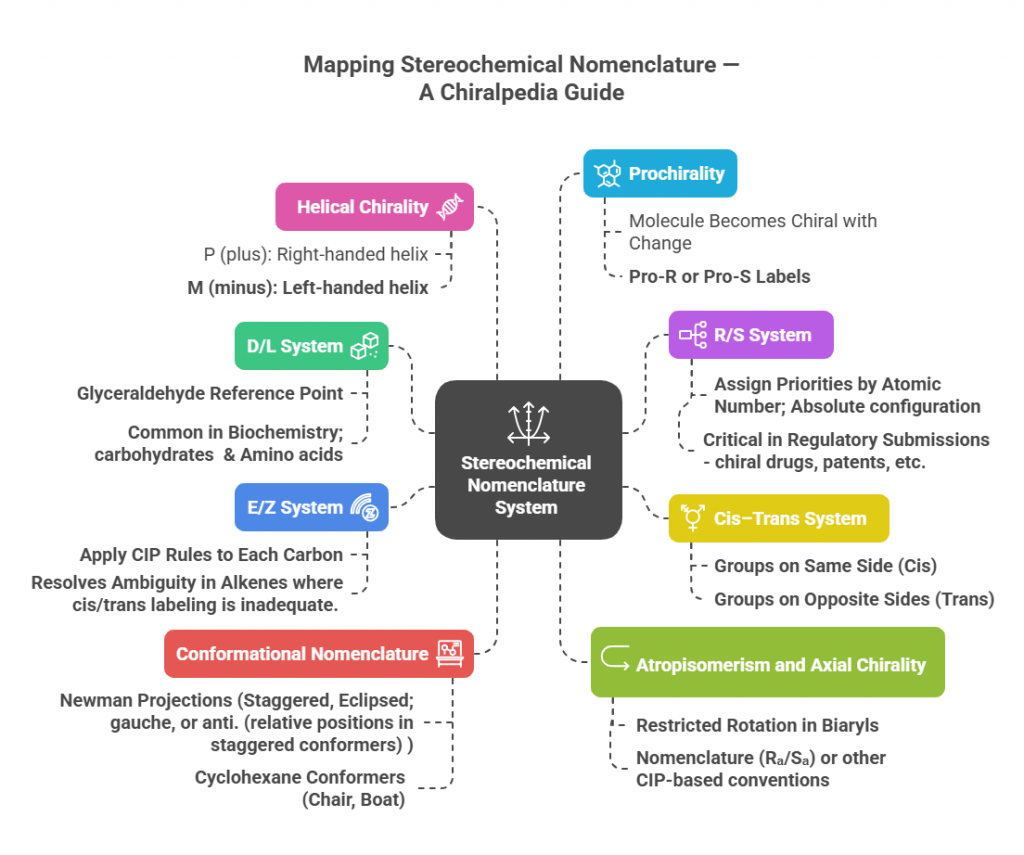
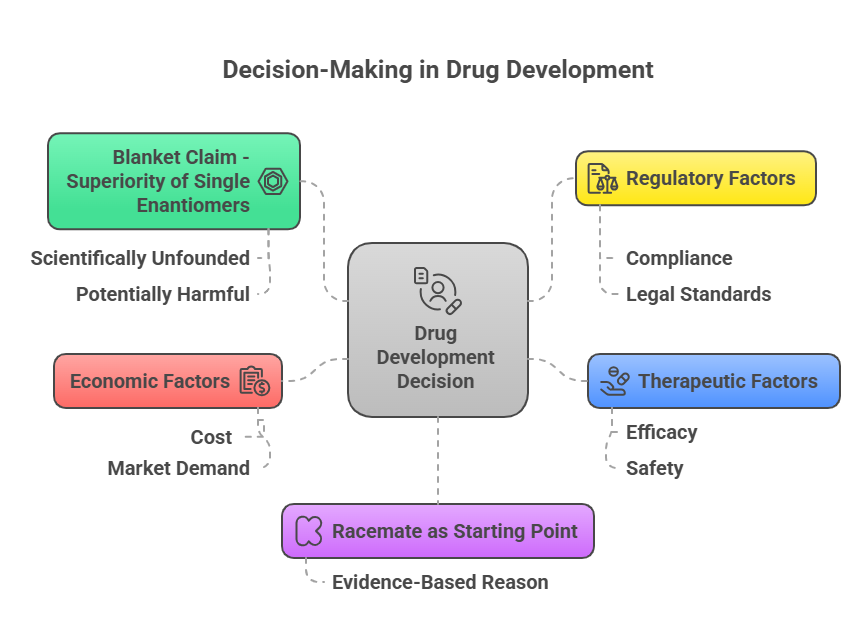
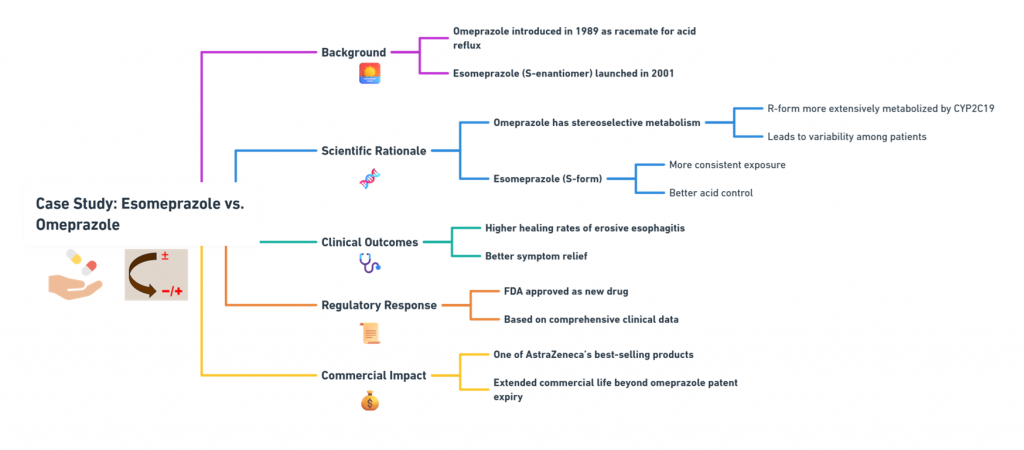
“Decoding the rules: how chemists name and navigate molecular twists.” Introduction Correctly describing the stereochemistry of a molecule is as important as understanding it. In this part, we focus on the Cahn–Ingold–Prelog (CIP) system, which provides the rules for unambiguous assignment of absolute configuration at stereocenters (R/S) and for double bond geometry (E/Z) system. We will outline the CIP priority rules step-by-step and demonstrate how to apply them to pharmaceutical molecules. Additionally, we will discuss …