Many medicinal agents important to life are combinations of mirror-image twins called chiral compounds. These twins may look very similar, yet their biological makeup might differ significantly. In other words, the pharmacokinetic and pharmacodynamic profiles of the individual enantiomers that make up a racemic chiral medication might drastically vary. The Chiral Molecule of the Week #cMOTW is an effort to highlight significant chiral compounds with therapeutic value. Follow us for more ……
Nature is not even handed. Unichirality – is hallmark of many molecules from nature.

The opioid agent morphine is a millennia-old-unichiral drug; Opium powder is the dried juice from the unripe seed capsule of the poppy Papaver somniferum; analgesic and euphoric.
Chirality and biological activity
Morphine has a highly demanding chemical structure. It is a pentacyclic 3°amine (alkaloid) with 5 stereogenic centers and exists in 32 stereoisomeric forms (2n = 25= 32 stereoisomers; n being number of stereogenic centers). But the desired analgesic activity resides exclusively in the natural product, the (-)-enantiomer with the configuration ((5R,6S,9R,13S,14R). (In the structure below stereogenic carbons are numbered in red font and configuration assigned in brackets.)
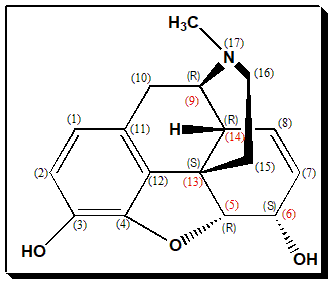
Nomenclature
7,8-didehydro-4,5-epoxy-17-morphinan-3,6α diol
It is interesting to note that when we humans try to synthesize chiral molecules, at large, we end up in racemic mixtures. To make enantiopure molecules one need to employ specialized tools viz. enantioselective catalysts/synthesis and for developing such innovative and breakthrough tools scientists are awarded Nobel prizes. For instance, Nobel prizes in Chemistry are awarded in 2001 (https://www.nobelprize.org/prizes/chemistry/2021/press-release/) and again in 2021, after a decade, (https://www.nobelprize.org/prizes/chemistry/2021/press-release/) for developing such ingenious tools But nature make enantiopure molecules elegantly, neatly, and silently in an eco-friendly environment with so much ease. Nature is incredible!!!.
Therapeutic category
Narcotic analgesic and sedative
Exercise
Construct molecular model of morphine and learn to assign absolute configuration to the stereogenic centers as per CIP rule.
References
Joseph Gal in Chirality in drug research. Edited by Eric Francotte, W. Lindner. Weinheim: Wiley-VCH. Page 3-25, 2006. ISBN 978-3-527-60943-7.
Wainer, l. W., & Drayer, D. E., Eds. Drug stereochemistry and Pharmacology: Marcel-Dekker, New York,1988.
Ernest L. Eliel Samuel H. Wilen, Stereochemistry of Organic Compounds, John Wiley and sons, New York.1994. ISBN 0-471-01670-5
Chiral drugs. Wikipedia, Wikipedia Foundation, 27/01/2022. https://en.wikipedia.org/wiki/Chiral_drugs
“The handed world“, https://chiralpedia.com/blog/the-handed-world/.
“Chiral twins – Identical? … But not really!”, https://chiralpedia.com/blog/ chiral-twins-identical-but-not-really/.
Harkishan Singh and V.K. Kapoor. Medicinal chemistry and pharmaceutical chemistry, Vallabh Prakashan, New Delhi, 2012.

Dear sir,
Thank you for sharing such an useful information on morphine, how it’s stereochemistry influence activity,
Dear Rajini,
You may read the following blog. https://chiralpedia.com/blog/chiral-twins-identical-but-not-really/. That might throw some light on this.
Hi Prof,
cMOTW is a laudable effort which would help chiral enthusiats get to know more about chiral pharmacology. Looking forward for morel
Thanks
Dear Dr.Chandramouli,
Thanks for the encouraging words. Morphine is the first chiral molecule in the series. cMOTW is intended to cover a wide range of chiral molecules.
Best regards, Valliappan