Esomeprazole is used to treat conditions where there is too much acid in the stomach. It is used to treat duodenal and gastric ulcers, erosive esophagitis, gastroesophageal reflux disease (GERD), and Zollinger-Ellison syndrome, a condition wherein the stomach produces too much acid. It was launched by AstraZeneca, in 2000. Esomeprazole magnesium [AstraZeneca; http://www.astrazeneca.com] (gastrointestinal).
Chirality and biological activity
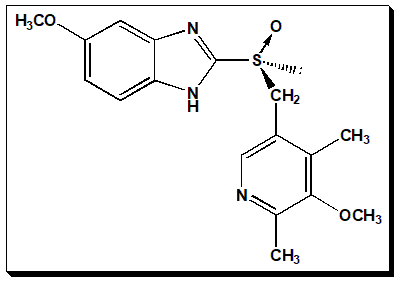
Esomeprazole is the (S)-(−)-enantiomer of (±)-omeprazole. It is a chiral switch (Read more about chiral switch @ my page in Wikipedia – <Chiral switch. Wikipedia, Wikipedia Foundation, 22/07/2022. https://en.wikipedia.org/wiki/Chiral_switch>.) Its chirality stems from the presence of stereogenic center at the sulfur atom of methylsulfinyl bridge between 1H-benzimidazole and the pyridine moieties.
In commercial terms, probably the most important chiral switch so far has been the switch from the blockbuster gastric anti-secretory proton pump (H+/K+-ATPase) inhibitor (PPI) omeprazole to esomeprazole. (±)-Omeprazole is a racemate launched in 1988 by Astra AB was the world’s highest-selling drug, and had worldwide annual sales of US $6.2 billion in 2000.
Claims for the switch from (±)-Omeprazole to S-Omeprazole include:
- Less variable metabolism; Esomeprazole clearance is less dependent on CYP2C19 than the racemate
- Higher dose efficiency – S-isomer 4 x better in preventing acid secretion than R-isomer
- Lower first pass metabolism
- Slower plasma clearance and increased systemic availability compared to R- isomer;
- Increased clinical efficiency
Nomenclature
(S)-(-)- 5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl}-1H-benzimidazole
Therapeutic category
Proton pump inhibitor; Treatment of peptic ulcer disease
Exercise
- Identify the stereogenic center in Esomeprazole
- Number the ring system and examine the nomenclature
References
Israel Agranat, Hava Caner and John Caldwell, Nature reviews, Drug Discovery, 1, October,| 753-768, 2002.
Hans-Jürgen Federsel in Chirality in drug research. Edited by Eric Francotte, W. Lindner. Weinheim: Wiley-VCH. ISBN 978-3-527-60943-7. Page 39 2006.
Chiral switch. Wikipedia, Wikipedia Foundation, 22/07/2022. https://en.wikipedia.org/wiki/Chiral_switch
