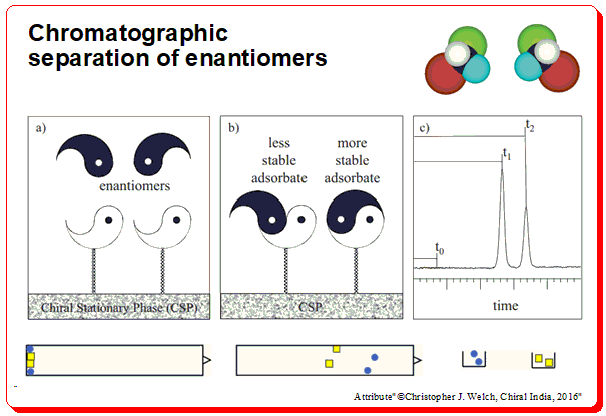
Chiral stationary phases (CSPs)
The most convenient and most popular analytical methodology to assess chiral purity is the direct separation of enantiomers on so-called chiral stationary phases (CSPs). CSPs consists of an (ideally) inert chromatographic support ( usually silica micro-particles) on to which the chiral selector (CS; single enantiomer of a chiral molecule) is physically coated (adsorption) or immobilization (covalently linked).
Basic concept
The column is packed with a chiral selector (viz. polysaccharide or protein or cyclodextrin, etc). You are loading the chiral analyte/sample to be separated from the top. As the sample travels through the CSP it forms a transient diastereomeric complex with the chiral selector. It gets separated based on the energy difference between the diastereomeric complexes. A schematic diagram to understand the concept of direct chiral HPLC separation is depicted below.
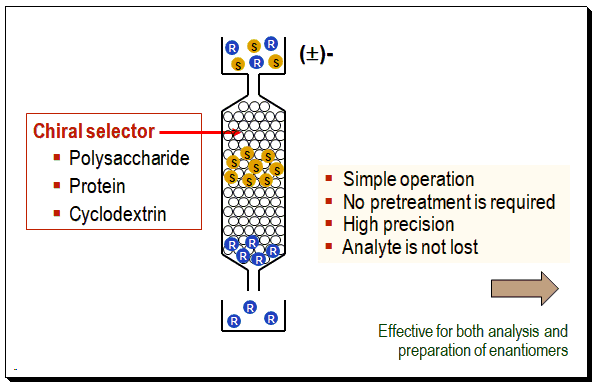
Mechanism of Chiral recognition
Chiral recognition implies the ability of chiral stationery phases to interact differently with mirror-image molecules, leading to their separation. The mechanism of enantiomeric resolution using CSPs is generally attributed to the “three-point” interaction model between the chiral analyte (CA) and the chiral selector (CS) in the stationary phase. The thermodynamic principle of chiral recognition is schematically represented below.
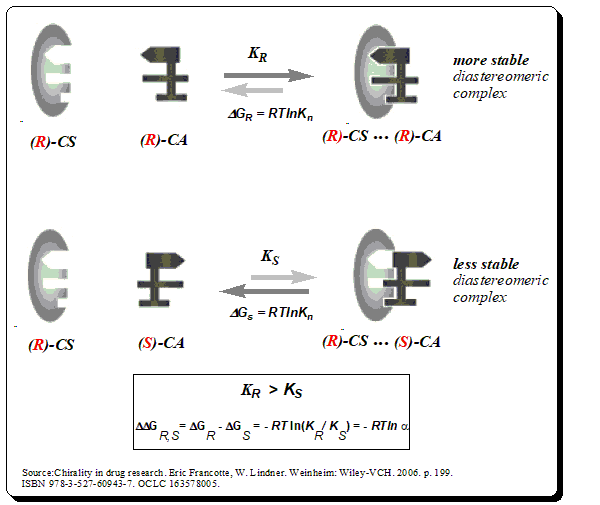
Under this model, for chiral recognition, and hence enantiomeric resolution to happen on a CSP one of the enantiomers of the analyte must be involved in three simultaneous interactions. This means to say the one of enantiomers is able to have a good interaction with the complimentary sites on the chiral selector attached to the CSP while its mirror-image partner may only interact at two or one such sites.
The diastereomeric complexes thus formed will have different energies of interaction. The enantiomer forming the more stable complex will have less energy and stay longer in the stationary phase compared to the less stable complex with higher energy. The concept is graphically depicted below for better understanding.
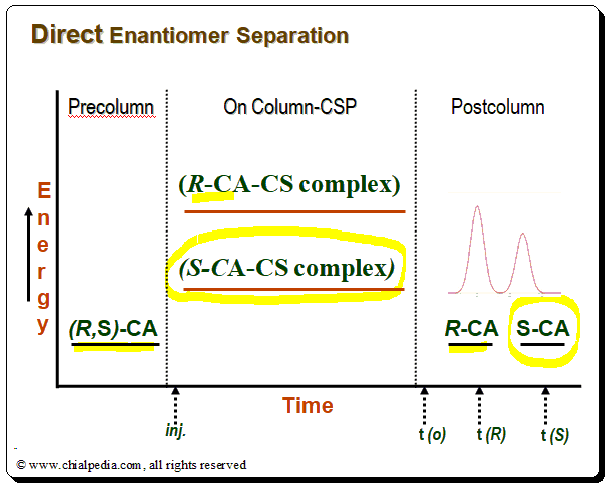
- Temporary diastereomeric complexes is formed between the enantiomeric analyte and immobilized chiral selector (in the column) [(R-CA-CSP complex) and (S-CA-CSP complex)]
- The energy difference between the complexes, E(R-CA-CSP complex) – E(S-CA-CSP complex), reflects the magnitude of enantioselectivity
- Energy of the sum total of interactions between analyte and CSP determine the observed retention time and efficiency
The success of chiral separation basically depends in manipulating the subtle energy differences between the reversibly formed non-covalent transient diastereomeric complexes.
MP role in Chiral Recognition events
The above discussed simplified bimolecular interaction model is a treatment suitable for theoretical purposes. It should be understood that the Mobile phase has a major role in stabilizing the diastereomeric complex and thus in chiral separation. Mobile phase plays a key role in chiral recognition mechanism. Components of MP (such as bulk solvents, modifiers, buffer salts, additives) not only influence the conformational flexibility of CS and CA molecules but also their degree of ionization. The types of interaction involved in the analyte-selector interaction vary depending on the nature of the CSP used. These may include hydrogen bonding, dipole-dipole, π-π, electrostatic, hydrophobic or steric interactions, and inclusion complex formation.
Further reading
Francotte, Eric; Lindner, Wolfgang (2006). Chirality in drug research. Eric Francotte, W. Lindner. Weinheim: Wiley-VCH. ISBN 978-3-527-60943-7. OCLC 163578005.
Thomas E. Beesley, Review of Chiral Stationary Phase Development and Chiral Applications, LCGC Europe, 05-01-2011, Volume 24, Issue 5, Pages: 270–276
Teixeira, Joana; Tiritan, Maria Elizabeth; Pinto, Madalena M. M.; Fernandes, Carla (2019-02-28). “Chiral Stationary Phases for Liquid Chromatography: Recent Developments”. Molecules. 24 (5): 865. doi:10.3390/molecules24050865. ISSN 1420-3049. PMC 6429359. PMID 30823495.
Christopher J. Welch, Reflections on Chirality in the Pharmaceutical Industry: Past, Present and Future, CHIRAL INDIA, 2016, November, Mumbai. India.
Chiral analysis. Wikipedia, Wikipedia Foundation, 05/11/2022. https://en.wikipedia.org/wiki/Chiral_analysis