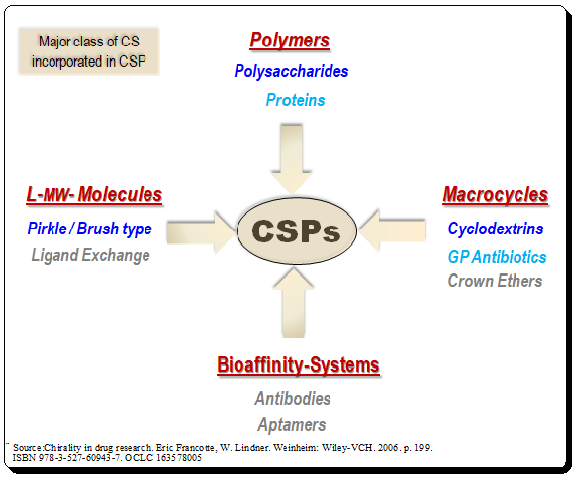
Lead
A wide range of chiral compounds have been examined as chiral selectors with regard to their ability to separate enantiomers using chromatography as part of the development of CSPs. From low-molecular-weight chemicals to polymers with both synthetic and biological origins, the chiral molecules investigated as potential CSs span almost the full spectrum of chemical and structural diversity. The search for effective CSs has so far led to the synthesis of more than 1400 CSPs, the characteristics of which are described in a virtually limitless number of specialized scientific papers. These efforts have resulted in an incredibly comprehensive toolset of more than 200 commercially available CSPs provided by several specialist suppliers. The purpose of this page is to introduce newcomers to the CS systems included in the most popular classes of commercial CSPs.
Types
For convenience and understanding purpose chiral selectors may be categorized into two types namely classical and novel chiral selectors. Classical and the more popular CSs include polysaccharides, cyclodextrins, proteins, glycopepetide, etc. Under the novel chiral selectors we can include chitosan, cyclofructan and chiral porous materials
. Utilizing structure-specific characteristics and the molecular weight range as classification criteria, CS systems incorporated in CSP may be divided into four main groups to offer a systematic framework. These groups include polymers, macrocycles, low-molecular weight scaffolds and bioaffinity systems as chiral selectors (CSs). The most widely employed CSs are presented in the figure below. Detailed discussion about the CSP based on these CS will be presented in the forthcoming #chiral_chromatography series.
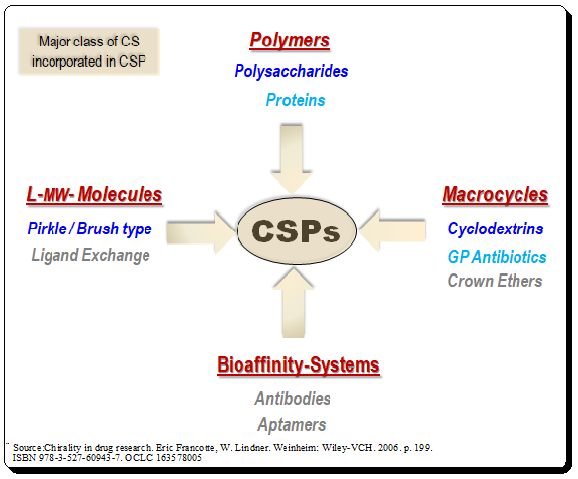
An overview of the basic nature of the most popular CSs are addressed in this context.
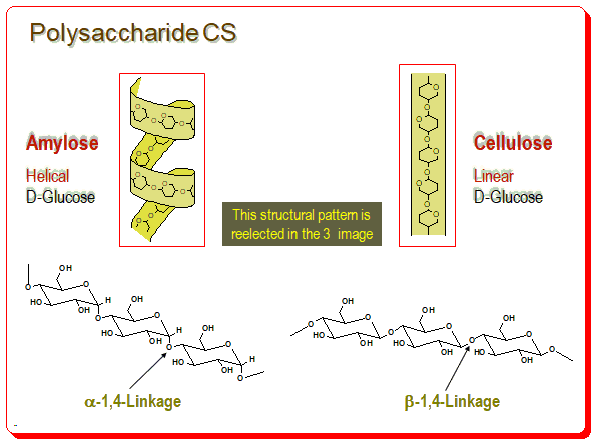
Polysaccharide
Polysaccharides are the most abundant chiral polymers on earth. These naturally occurring polysaccharides form basis for an important class of CS. Basically there are problems in handling and poor resolution quality. Derivatives of these polymers, especially Amylose & Cellulose, exhibit excellent properties as CSP for HPLC.
Proteins
Proteins are complex, high-MW biopolymers. Composed of L-amino acids and possess ordered 3D-structure. Protein polymer remains in twisted form because of the different intramolecular bonding; these bonding are responsible for different type of chiral loops/grooves present in the protein molecule. Known to bind /interact stereoselectively with small molecules reversibly, making them extremely versatile CSPs for chiral separation of drug molecules. Number of CSPs has been developed by immobilizing proteins or enzymes.
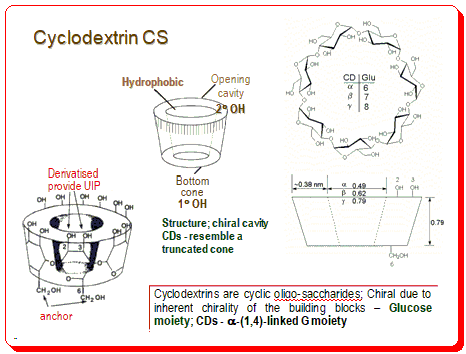
Cyclodextrins
Cyclodextrins are cyclic oligosaccharides. They are chiral due to inherent chirality of the building blocks – Glucose moiety. Commercially available cyclodextrins have 6 or 7 or 8 glucose units. They form an important class of CSs.
Low-molecular weight CS
Donor–acceptor-type CSPs capitalize on synthetic or semi-synthetic chiral low-molecular-weight CSs capable of enantioselectively recognizing analytes by complementary arrays of nonionic attractive interactions. The repertoire of these nonionic interactions generally comprises hydrogen bonding, p-p-stacking, dipole-dipole-stacking and steric interactions. The majority of donor–acceptor-type CSs has been designed to exploit p-p-stacking interactions between electron-rich and electron-deficient aromatic systems as the primary attractive interaction force.
Further reading
Francotte, Eric; Lindner, Wolfgang (2006). Chirality in drug research. Eric Francotte, W. Lindner. Weinheim: Wiley-VCH. ISBN 978-3-527-60943-7. OCLC 163578005.
Thomas E. Beesley, Review of Chiral Stationary Phase Development and Chiral Applications, LCGC Europe, 05-01-2011, Volume 24, Issue 5, Pages: 270–276
Teixeira, Joana; Tiritan, Maria Elizabeth; Pinto, Madalena M. M.; Fernandes, Carla (2019-02-28). “Chiral Stationary Phases for Liquid Chromatography: Recent Developments”. Molecules. 24 (5): 865. doi:10.3390/molecules24050865. ISSN 1420-3049. PMC 6429359. PMID 30823495.
Christopher J. Welch, Reflections on Chirality in the Pharmaceutical Industry: Past, Present and Future, CHIRAL INDIA, 2016, November, Mumbai. India.
Chiral analysis. Wikipedia, Wikipedia Foundation, 17/11/2022. https://en.wikipedia.org/wiki/Chiral_analysis