Strategy
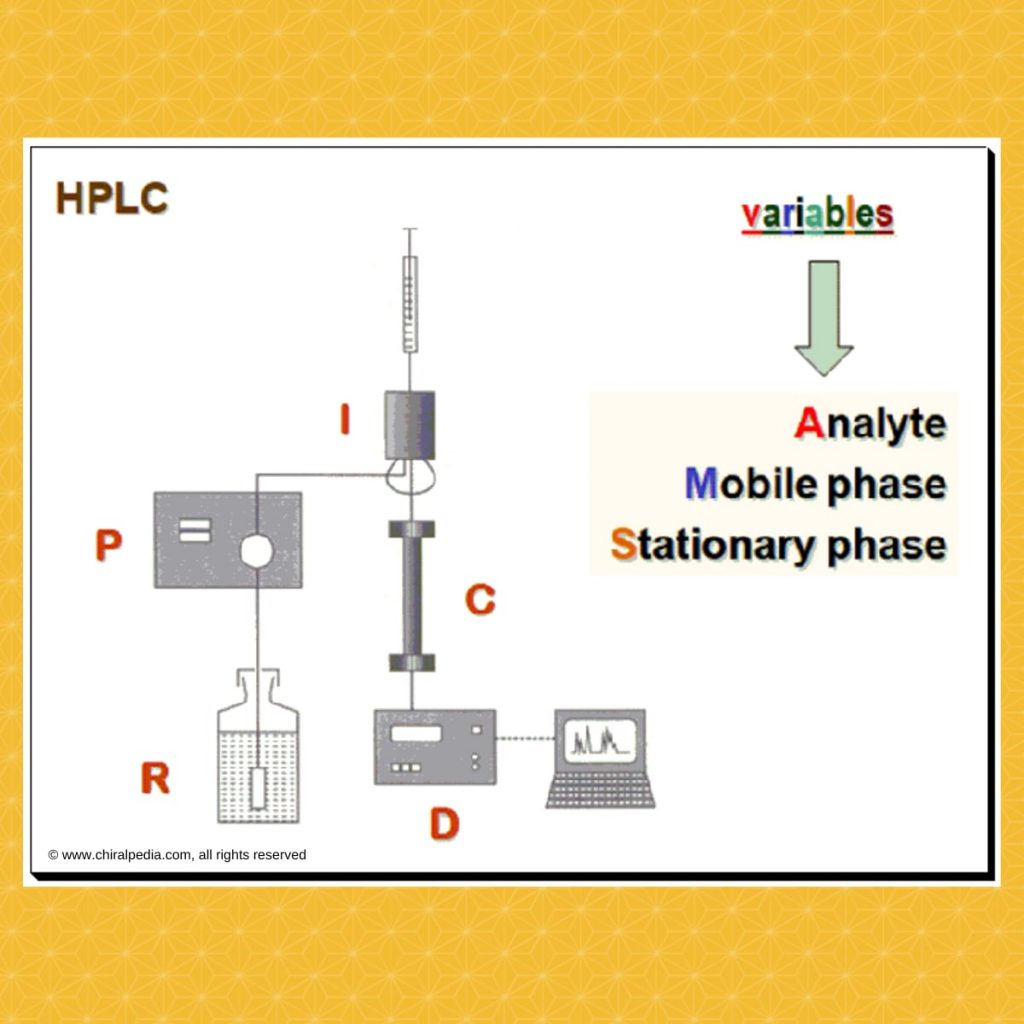
The basic aspect is to provide the right chiral environment where by the enantiomeric pair become distinguishable. In HPLC how do we construct the chiral environment? To understand this let us look at the variables available, in the HPLC system, that can be exploited for this purpose. The following is a schematic diagram of a typical HPLC system. The are three variables namely the analyte, mobile phase and stationary phase that can be alerted to construct the required chiral environment.
Approaches
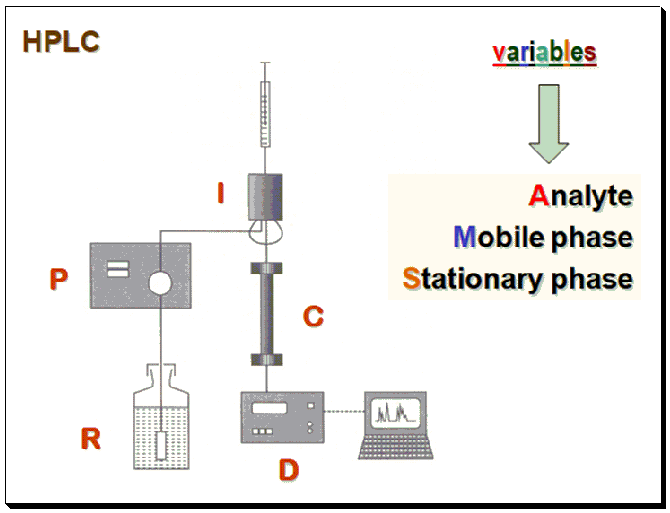
These variables are made to interact with a chiral auxiliary (chiral selector, CS) whereby it forms a diastereomeric complex which has different physicochemical properties and makes it possible to separate the enantiomers.
1. Analyte – Derivatize using CS ( here – Chiral Derivatization Agent, CDA)
2. Stationary Phase – Attach/immobilize CS to silica (here, chiral stationary phase)
3. Mobile Phase – Incorporate CS (here – chiral mobile phase additive, CMPA)
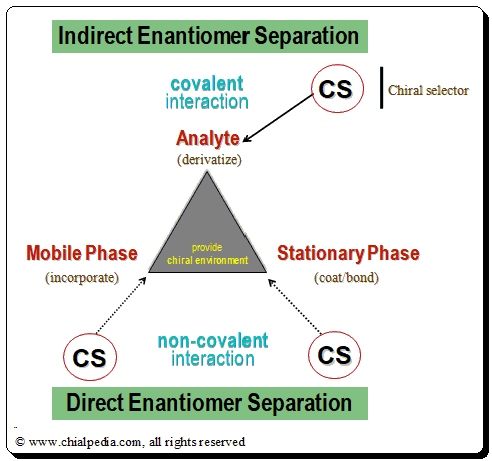
Based on the nature of the diastereomeric complex formed between the CS-CA species, enantiomer separation methodologies are categorized into approaches: Indirect and direct enantiomer separation mode.
Indirect Enantiomer separation
The chiral analyte (CA) of interest interacts with the appropriate reactive CS (in this case, an enantiopure chiral derivatizing agent, CDA), resulting in the formation of a covalent diastereomeric complex that can be separated using an achiral chromatographic method. This process is known as indirect enantiomer separation. Reactive functional groups (amino, hydroxyl, epoxy, carbonyl, carboxylic acid, etc.) are frequently found in the structures of therapeutic medicines. With the aid of an enantiomerically pure chiral derivatizing agent, they are transformed into covalently bound diastereomeric derivatives. The success of this method depends on the availability of stable enantiopure chiral derivatizing agent (CDA) and on the presence of an appropriate reactive functional group in the chiral drug molecule for covalent formation of diastereomeric species.
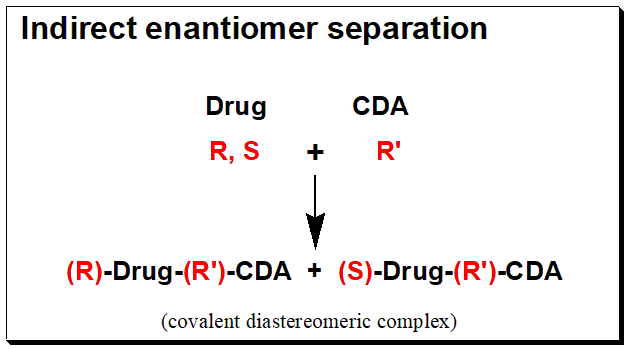
The reaction of a racemic, (R,S)- Drug with a chirally and chemically pure chiral derivatizing agent, (R’)-CDA, will afford diastereomeric products, (R)-Drug-(R’)-CDA + (S)-Drug-(R’)- CDA. The box below shows an illustration of the chiral derivatization reaction scheme.
Diastereomers, as opposed to enantiomers, differ in their physicochemical characteristics and can be separated on conventional achiral stationary phases. The main advantage of the indirect methodology is that the generated diastereomers can be separated using a conventional achiral stationary phase/mobile phase system. Thus, chromatographic conditions can be quite flexible in order to achieve the desired separation and to get rid of endogenous and metabolite interferences. Furthermore, wise selection of the CDA and the chromatographic detection system can increase the method’s sensitivity. This indirect method of enantiomeric analysis, however, may have some drawbacks. There must be a suitable functional group on the enantiomer for derivatization, the CDA must be pure in both enantiomers, the CDA must not racemize during derivatization, and the analyte must not racemize during derivatization. However, the use of indirect analytical methods is currently decreasing.
Direct separation of enantiomers
The chiral selector/discriminator and the analyte (drug enantiomer) form a transient rather than covalent diastereomeric complexation during direct enantiomer separation. In this method, the chiral recognition is accomplished by taking advantage of the minute energy differences existing between the reversibly formed noncovalent diastereomeric complexes. There are two methods for achieving direct chromatographic enantiomer separation: chiral mobile phase additive and chiral stationary phase mode
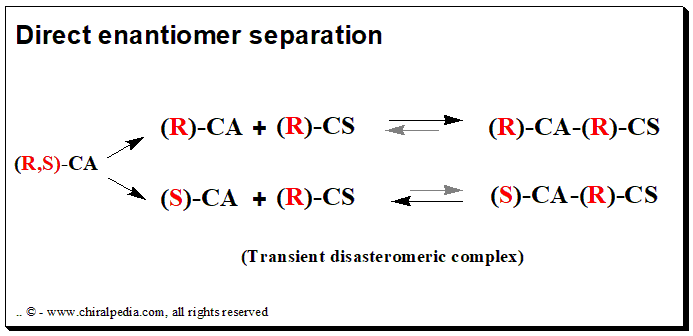
Chiral mobile phase additive (CMPA)
In this method, the mobile phase is incorporated with an enantiomerically pure compound, the chiral selector, and separation takes place on a typical achiral column. Individual enantiomers form transient diastereomeric complexes with the chiral mobile phase additive when a mixture of enantiomers is added to the chromatographic system.
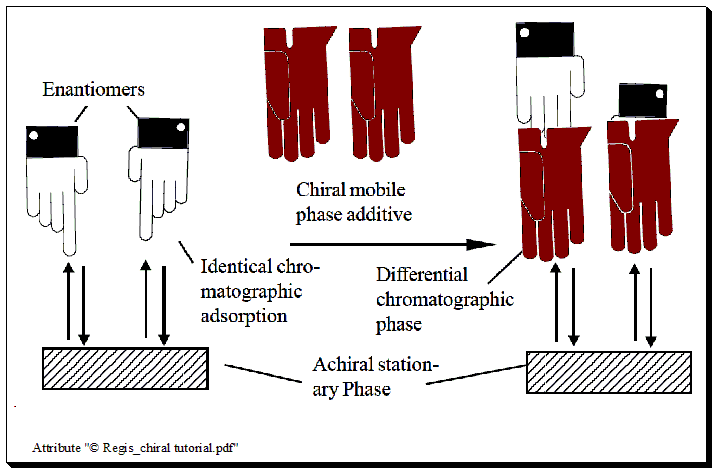
Two potential mechanisms could be at work in the chiral mobile phase additive technique: one is that the enantiomers and CMPA could form diastereomers in the mobile phase. One more is the possibility of coating the stationary phase with CMPA, which would cause diastereomeric interactions with the enantiomeric pairs to occur during the chromatographic separation process. According to the characteristics of the stationary phase and mobile phase used, both mechanisms may occur. This method has recently found limited use.
Chiral stationary phase (CSP)
The most common method for direct enantiomer separation involves the use of chiral stationary phases. The stationary phase is where the chiral selector is located in this instance. The chiral stationary phase is made up of a single enantiomer of a chiral molecule (the selector), which is coated, adsorbed, or chemically linked to the surface of an inert solid support (typically silica microparticles). Proteins, cyclodextrins, polysaccharides, and other substances are frequently used chiral selectors.
Further reading
Linder, W (1988). “Indirect separation of enantiomers by liquid chromatography”. Chromatographic Science Series. 40: 91–130.
Francotte, Eric; Lindner, Wolfgang (2006). Chirality in drug research. Eric Francotte, W. Lindner. Weinheim: Wiley-VCH. ISBN 978-3-527-60943-7. OCLC 163578005.
Thomas E. Beesley, Review of Chiral Stationary Phase Development and Chiral Applications, LCGC Europe, 05-01-2011, Volume 24, Issue 5, Pages: 270–276
Teixeira, Joana; Tiritan, Maria Elizabeth; Pinto, Madalena M. M.; Fernandes, Carla (2019-02-28). “Chiral Stationary Phases for Liquid Chromatography: Recent Developments”. Molecules. 24 (5): 865. doi:10.3390/molecules24050865. ISSN 1420-3049. PMC 6429359. PMID 30823495.