Lead
Cyclodextrin-based CSP belongs to the cavity type. Armstrong is considered the father of cyclodextrin-based CSPs. CDs are employed in various fields of analytical chemistry, due to their tendency to form reversible inclusion complexes and recognize chiral analytes. CDs are a type of macrocyclic D-(+)-glucopyranose oligomers linked by α-1,4-glycosidic linkages, as seen in Figure, under chemistry . The relevant oligomers for liquid chromatographic enantiomer separation are composed of 6(α-CD), 7(β-CD), and 8(γ-CD) monosaccharide units, in that order.
CDs have a long history as adaptable host systems in the field of molecular recognition and have been widely utilized in a variety of enantiomer separation techniques, including GC, LC, CE, and CEC. Recent reviews detail the numerous applications of CDs for enantiomer separation of chiral medicinal molecules.
Chemistry
Cyclodextrins are cyclic oligo-saccharides and are chiral due to inherent chirality of the building blocks – Glucose moiety; CDs – a-(1,4)-linked glucose moiety. The overall conformations of CDs resemble truncated cones, with hydrophobic methylene groups and glycosidic ether oxygens lining the inside. The wider and narrower rims of CDs are adorned with secondary and primary hydroxyl groups, respectively, rendering these macrocycle regions hydrophilic. CD structure is depicted in the image below demarking the inner hydrophobic and outer (rim) hydrophilic regions.
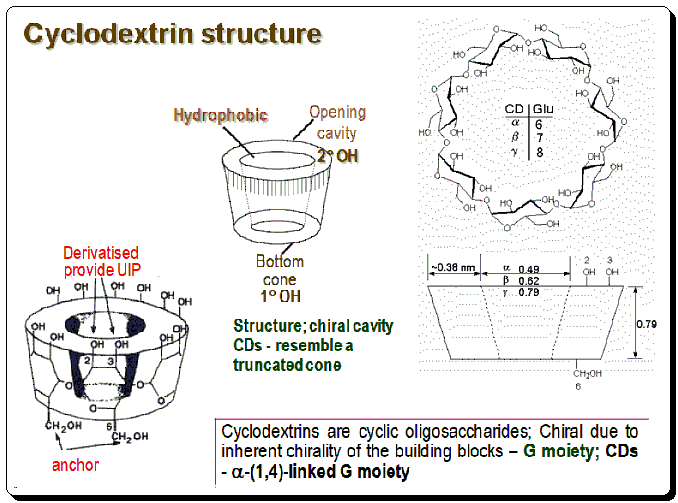
Preparation
Both coated and immobilized CD-based CSPs are commercially available. Various strategies viz. the pioneering Armstrong process, click chemistry technique, etc., are employed for the fabrication of CD-based CSPs. For detailed discussion on the various preparation techniques the readership may consult books and articles cited in the section “Further reading”.
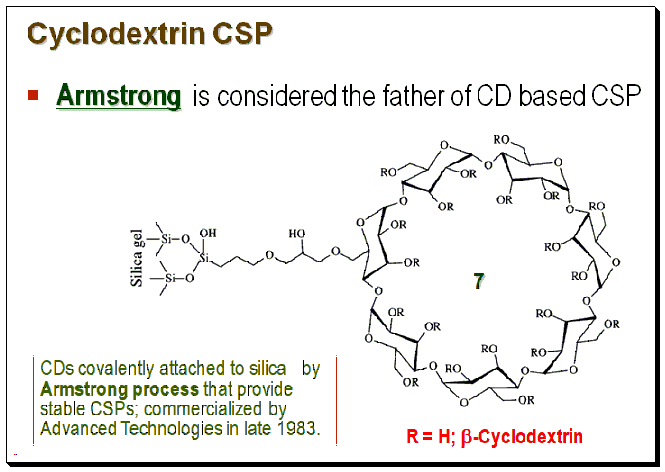
Mechanism
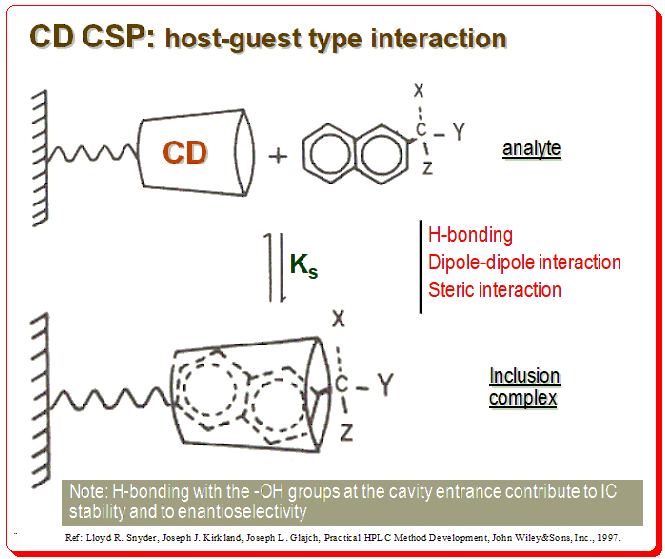
The CD molecules in these CSPs are often covalently bonded to functionalized silica surfaces via one or more of the main hydroxy groups, orienting the CD molecule so that the larger rim faces the solution. In addition to CSPs containing native CDs, different variants of enantioselective adsorbents have been produced. These extra functions may have supporting effects on chiral recognition by enlarging the chiral cavity and supplying additional interaction sites. The diagram below depicts CD molecule, as a truncated cone linked through a spacer to silica support matrix, which forms a reversible inclusion complex with a chiral naphthyl compound.
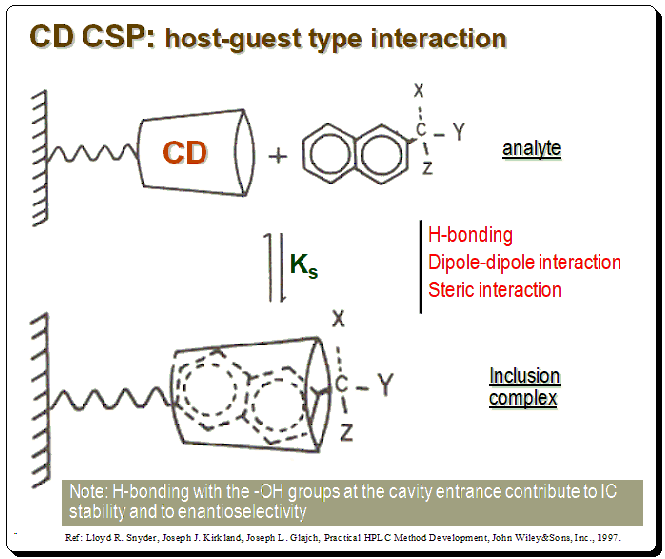
There are specific structural characteristics that assist chiral recognition to happen in CD based CSPs that include:
- Analyte size: Analyte molecule need to “fit” into the CD cavity; fit-unfit geometric parameter
- Analyte structure: An aromatic ring or cycloalkyl ring should be attached near stereogenic center of the analyte
- Interaction bet. CD and analyte: Substituents at or near the analyte chiral center must be able to interact with the –OH groups at the entrance of the CD cavity
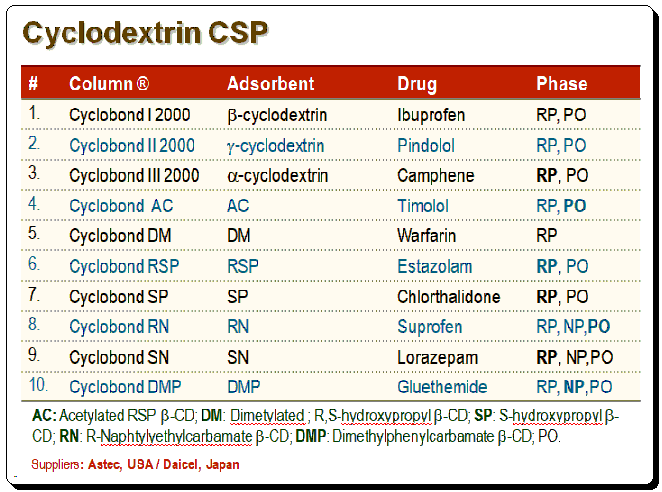
CD-based CSPs
Various bonded CD-type CSPs have been developed and commercialized for use in liquid chromatography. Table below provides a summary of the brand names, CD derivative type, and providers of these CSPs.
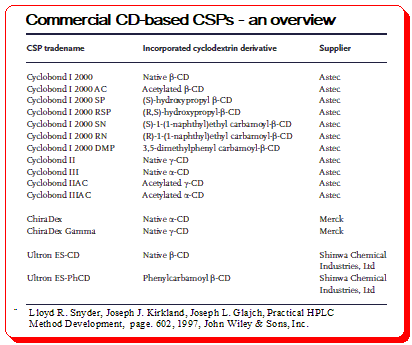
Application of CD-based CSPs
Detailed view of the application consult application guides/catalogues of manufacture/company viz. Daicel, Phenomenex, Regis, and etc. Also consult books given in the section “Further reading”.
Strength CD-based CSPs
- Aqueous compatibility of CD & its unique molecular structure make the CD-bonded phase highly promising for use in chiral separation of drugs
- Relatively cheaper than other CSP
- RP conditions are applied with CD CSP
Weakness of CD-based CSPs
- Limited to compounds that can enter into CD cavity
- Cannot invert elution order
- Small changes in analyte structure leads to unpredictable effect on resolution
- Often poor efficiency
Disclaimer
Due to the field’s quick development, there may have been some unintentional omissions or inadequate technical specifications. If any omissions or updates needing to be made are added, we would be pleased to include them. To draw attention to it, kindly write a comment. All technical specifications found here are reproduced as-is from the manufacturer’s literature and might have changed at the time of publication. All information given here is for furthering science and cannot be construed as professional advice.
Further reading
Lloyd R. Snyder, Joseph J. Kirkland, Joseph L. Glajch, Practical HPLC Method Development, pages. 600-615, 1997, John Wiley & Sons, Inc. ISBN 0-471-00703-X
Lajos Szente and Julianna Szemán, Cyclodextrins in Analytical Chemistry: Host–Guest Type Molecular Recognition, Anal. Chem. 2013, 85, 17, 8024–8030, https://doi.org/10.1021/ac400639y
Carla Fernandes, Joana Teixeira, Madalena M. M. Pinto, and Maria Elizabeth Tiritan, Strategies for Preparation of Chiral Stationary Phases: Progress on Coating and Immobilization Methods, Molecules. 2021 Sep; 26(18): 5477.DOI: 10.3390/molecules26185477
Alain Berthod (Editor), Chiral Recognition in Separation Methods: Mechanisms and Applications, 2010, Springer Heidelberg Dordrecht London New York, ISBN 978-3-642-12444-0, DOI 10.1007/978-3-642-12445-7
Hassan Y. Aboul-Enein, Imran Ali, Chiral Separations by Liquid Chromatography And Related Technologies, 2003, Marel Dekker Inc. ISBN: 0-8247-4014-9
Thomas E. Beesley, Review of Chiral Stationary Phase Development and Chiral Applications, LCGC Europe, 05-01-2011, Volume 24, Issue 5, Pages: 270–276
Teixeira, Joana; Tiritan, Maria Elizabeth; Pinto, Madalena M. M.; Fernandes, Carla (2019-02-28). “Chiral Stationary Phases for Liquid Chromatography: Recent Developments”. Molecules. 24 (5): 865. doi:10.3390/molecules24050865. ISSN 1420-3049. PMC 6429359. PMID 30823495.
Christopher J. Welch, Reflections on Chirality in the Pharmaceutical Industry: Past, Present and Future, CHIRAL INDIA, 2016, November, Mumbai. India.
Chiral analysis. Wikipedia, Wikipedia Foundation, 17/11/2022. https://en.wikipedia.org/wiki/Chiral_analysis

Dear Dr.Chandramouli,
Happy you find the article informative. Thanks for supporting chiral science. Shall try to post more such articles.
Bes regards,