Lead
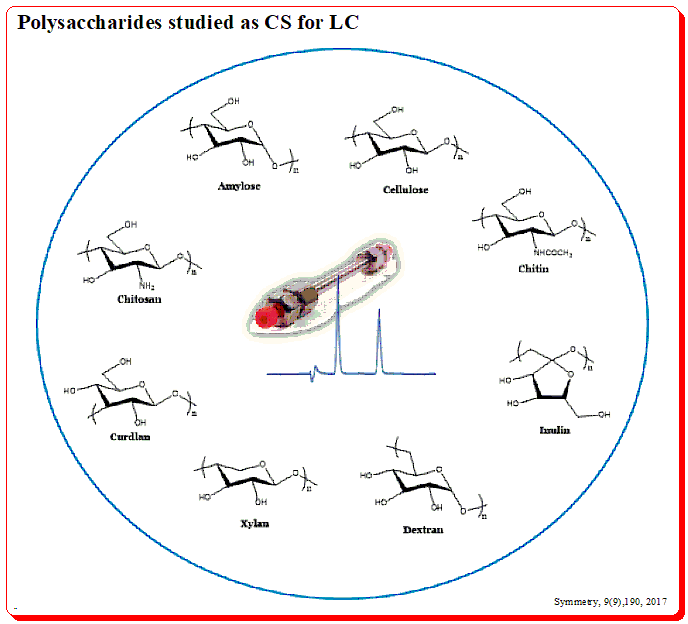
Surprisingly, chiral chromatography could not be performed in 1980 because there was no single chiral stationary phase on the market. But as the 1980s came to a close, chiral chromatography began to get more and more attention, thanks in large part to the efforts of Okamoto’s institute in Japan, Pirkle and Armstrong’s teams in the US, Schurig and König in Germany, Lindner in Austria, and Francotte in Switzerland. The most prevalent chiral polymers on earth are composed of the polysaccharides amylose and cellulose. These organic polysaccharides serve as the building blocks for a significant class of chiral selectors. (For a better understanding, consult the page on chiral selectors at www.chiralpedia.com/blog/chiralselectors/>.)
Landmarks in Polysaccharide-based CSPs development
Before going further let us look at the landmarks in the developments of polysaccharide-based CSPs. During the early studies of the chromatographic resolution of enantiomers, some polysaccharides and oligosaccharides were used as chiral adsorbents. Significant landmarks in the studies and development using polysaccharides and their derivatives are summarized in the table below.
| Year | Milestones |
| 1938 /1943 /1944 | Henderson & Rule; Lecoq; Prelog & Wieland on lactose |
| 1948 | Dent on cellulose (paper) |
| 1951 | Kotake et al., resolution by paper chromatography (cellulose) |
| 1952 | Dalgliesh on cellulose (paper) |
| 1954 | Krebs and Rasche on starch |
| 1957 | Musso on cellulose powder |
| 1966 | Lüttringhaus and Peters on acetylated cellulose |
| 1968 | Kratchanov and Popova on polygalacturonic acid |
| 1973 | Hesse and Hagel; microcrystalline cellulose triacetate |
| 1978 | Musso, resolution by potato starch |
| 1984 | Cellulose tribenzoate and trisphenylcarnbamate (Daicel, Okamoto); commercialization of cellulose-based CSPs (Daicel) |
| 1986 | Cellulose tris(3,5-dimethylphenylcarbamate) (Okamoto et al.) |
| 1987 | Amylose (tris(3,5-dimethylphenylcarbamate) (Okamoto et al.); immobilized CSP based on polysaccharide phenylcarbamate (okamoto et al.) |
| 2004 | Commercialization of immobilized CSP based on polysaccharide phenyl carbamate (Daicel) |
Chemistry
You find amylose has helical structure build up of D-glucose units linked in the α-1,4-format and cellulose having the linear structure due to the β-1,4-linkage of D-glucose units.
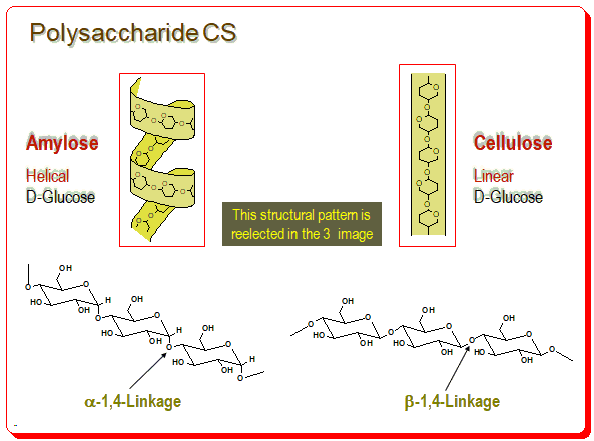
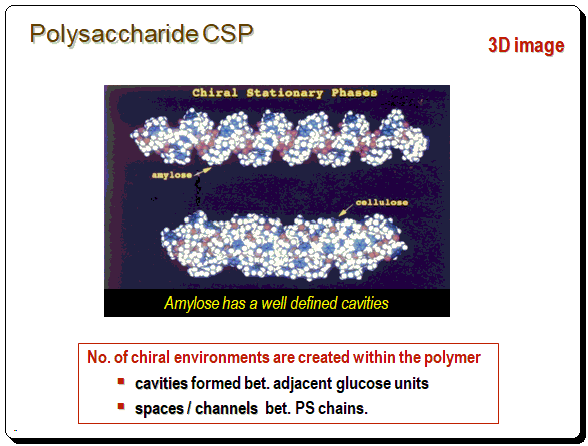
This structural pattern is evident in the 3D image as well. Number of chiral environments are produced within the polymeric structure called cavities (formed between adjacent glucose units) and spaces/or channels (formed between polysaccharide chains).
Polysaccharide CSPs is classified in two types namely the coated and immobilized based on the chemistry of the application of the CS to the chromatographic support matrix (usually silica). In the coated type, the polymeric chiral selector (amylose/cellulose dr.) is physically coated by an adsorption process where as in the immobilized type the CS is linked by chemical process.
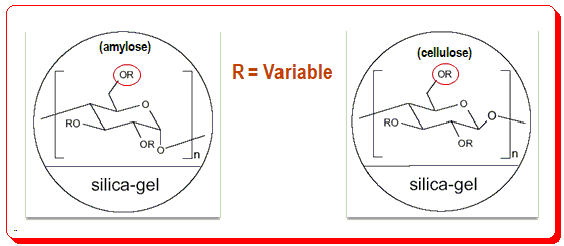
You find ‘R’ is the variable here and changing this factor results in different CSPs with varying chromatographic attribute and characteristics.
Chiral recognition mechanism
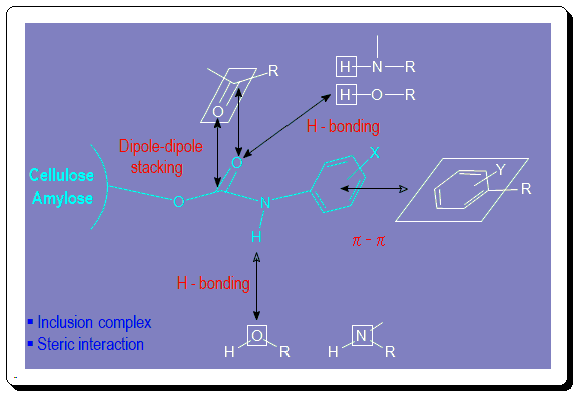
A detailed understanding of the mechanism of chiral recognition concerning chiral analyte interaction with polysaccharide-based CSP remains to be confirmed. Chiral recognition process has been proposed to involve a variety of interactions viz. dipole-dipole, steric, H-bonding, and steric interactions. In the image below cellulose and amylose tri(phenylcarbamate) derivatives interaction with different CA is depicted.
Preparation
Amylose and cellulose cannot be used as such since they are difficult to handle and have low resolution. However, these polymers’ carbamate and benzoate derivatives, particularly those from cellulose and amylose, have exceptional qualities as chiral selectors for chromatographic separation. High quality silica supports are used to create polysaccharide CSPs, which can either be physically coated (coated CSP) or chemically immobilized (immobilized CSP) with the polymeric chiral selector (amylose/cellulose derivative).
Coated polysaccharide CSPs
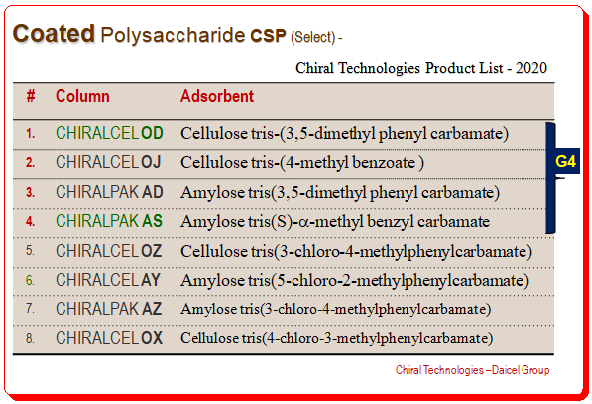
There are numerous commercially available CSPs for chiral separation that are polysaccharide-based. These CSPs demonstrated outstanding chiral recognition abilities to distinguish a variety of chiral analytes. Daicel Chemical Industries, Ltd. has sold many of these CSPs; a few of the more well-known ones are listed in the table. Many screening research studies conducted at different labs go to suggest that the four CSPs, namely Chiralcel OD, Chiralcel OJ, Chiralpak AD, and Chiralpak As, are capable of resolving more than 80% of the chiral separations due to their adaptability and high loading capacity. These four polysaccharide chiral stationary stationary phases are referred to as the “golden four” (indicated by “G4” in the table)
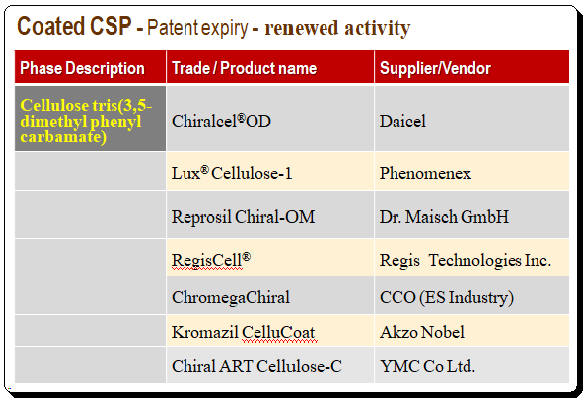
Coated Polysaccharide CSP – Patent expiry – renewed activity
When the patent for coated polysaccharide technology ran out, it led to a lot of new research and renewed activity from vendors and providers leading to competitive prices. A number of new clones of the popular cellulose and amylose derivatives have entered the field with a special emphasis on a 3-chloro-5-methylphenyl carbamate derivative of both cellulose and amylose. To illustrate this point cellulose tris-(3,5-dimethylphenylcarbamate) is taken as an example and details of product and supplier names are presented in the table below.
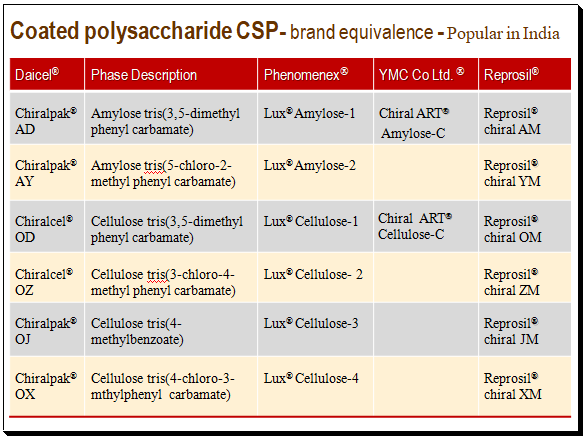
The following table present popular brands of coated polysaccharide CSPs in India
Weakness
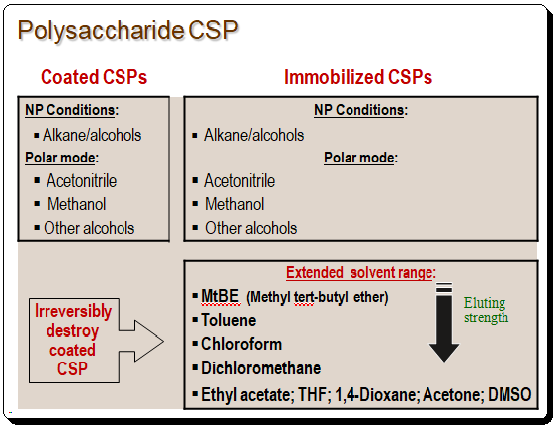
Polar organic mode, reversed-phase mode, and regular phase mode can all be used for separations. When working with coated polysaccharide CSP, caution must be taken when choosing the solvents. Extreme solvents like dichloromethane, chloroform, toluene, ethyl acetate, THF, 1,4-dioxane, acetone, DMSO, etc. shouldn’t be used. The stationary phase will be irrevocably destroyed by these so-called “non-standard” solvents, which will dissolve the silica and kill the stationary phase.
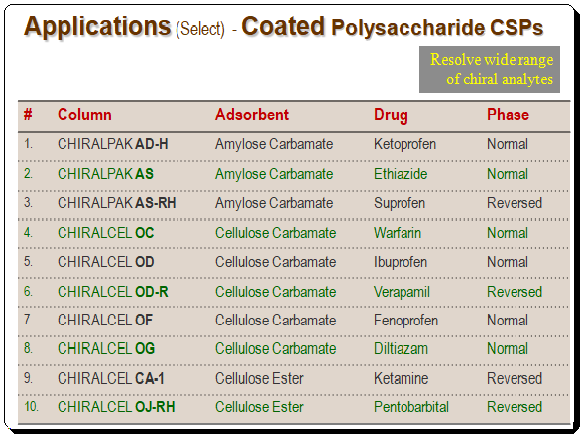
Applications of coated polysaccharide-based CSPs
Detailed view of the application consult application guides/catalogues of manufacture/company viz. Daicel, Phenomenex, Regis, and etc. Also consult books given in the section Further reading”.
Immobilized polysaccharide CSPs
The limited resistance of the coated polysaccharide CSPs to many solvents led to the development of immobilized polysaccharide CSPs. Thus expanding the choice of co-solvent. Immobilized CSPs are made with a silica support on to which the polymeric chiral selector (polysaccharide derivative) is immobilized. The table below lists some of the commercially available immobilized CSP along with the chemical nature of the CS.
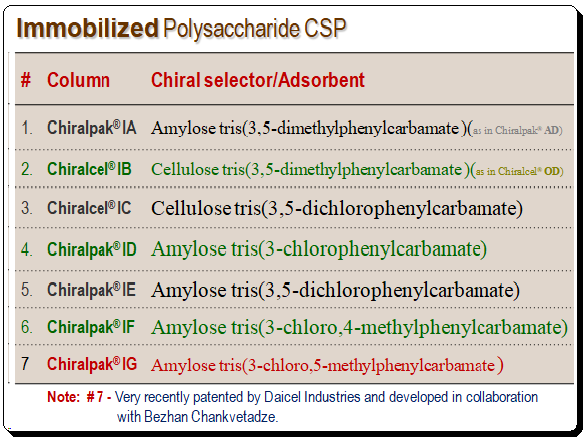
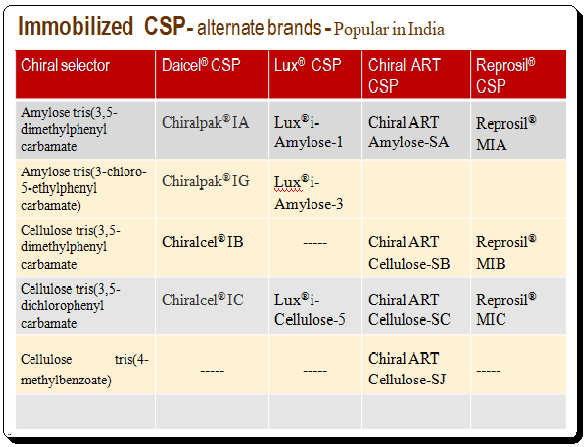
To get an overview of various brands of immobilized polysaccharide-based CSPs are presented below.
Strength
These immobilized CSP are far more durable and can be used with “non-standard” solvents increasing the available co-solvent options. Immobilized CSPs’ principal advantages are their great solvent versatility for choosing the mobile phase’s composition, improved sample solubility, strong selectivity, robustness and extended durability, outstanding column efficiency, and wide range of applications for enantiomer resolution. In HPLC method development solvent is a crucial component. Better sample solubility, improved resolution, and the ability to design efficient chiral methods are all made possible by having access to more solvents. Th following chat shows the solvent rage of coated and immobilized polysaccharide-based CSPs.
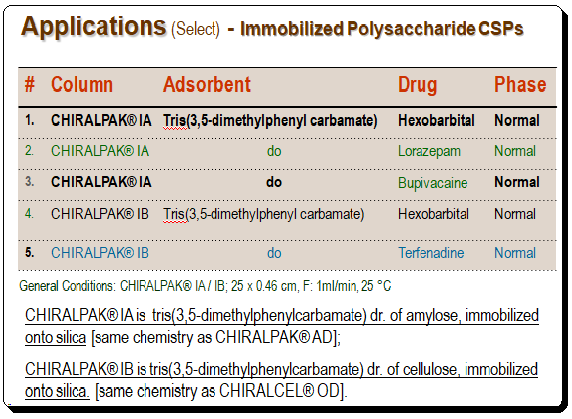
Applications of immobilized polysaccharide-based CSPs
A gist of the application of immobilized polysaccharide-type CSPs is presented in the table below. Detailed view of the application component is out of scope of the page and readerships may go through application guides/catalogues of manufacture/company viz. Daicel, Phenomenex, Regis, and etc. Also consult books given in the section “Further reading”.
Disclaimer
Due to the field’s quick development, there may have been some unintentional omissions or inadequate technical specifications. If any omissions or updates needing to be made are added, we would be pleased to include them. To draw attention to it, kindly write a comment. All technical specifications found here are reproduced as-is from the manufacturer’s literature and might have changed at the time of publication. All information given here is for furthering science and cannot be construed as professional advice.
Further reading
Francotte, Eric; Lindner, Wolfgang (2006). Chirality in drug research. Eric Francotte, W. Lindner. Weinheim: Wiley-VCH. ISBN 978-3-527-60943-7. OCLC 163578005.
Tomoyuki Ikai and Yshio Okamoto. Structure control of polysaccharide derivatives for efficient separation of enantiomers by chromatography, Chem. Rev.2009, 109, 6080. DOI: 10.1021/cr8005558
Thomas E. Beesley, Review of Chiral Stationary Phase Development and Chiral Applications, LCGC Europe, 05-01-2011, Volume 24, Issue 5, Pages: 270–276
Teixeira, Joana; Tiritan, Maria Elizabeth; Pinto, Madalena M. M.; Fernandes, Carla (2019-02-28). “Chiral Stationary Phases for Liquid Chromatography: Recent Developments”. Molecules. 24 (5): 865. doi:10.3390/molecules24050865. ISSN 1420-3049. PMC 6429359. PMID 30823495.
Christopher J. Welch, Reflections on Chirality in the Pharmaceutical Industry: Past, Present and Future, CHIRAL INDIA, 2016, November, Mumbai. India.
Chiral analysis. Wikipedia, Wikipedia Foundation, 17/11/2022. https://en.wikipedia.org/wiki/Chiral_analysis

Dear sir
This is informative article on chiral chromatography. Thank you for sharing the information sir
Dear Selvakumar,
Glad you found it informative.
Regards,