“Pharmacological studies confirm the high activity of triprolidine and the superiority of (E) over corresponding (Z) isomers as H, antagonist” ( Ref: – – – from “Wilson and Gisvold’s Textbook of organic medicinal and pharmaceutical chemistry, 2010”). “Triprolidine is 2-[(E)-1-(4-methylphenyl)-3-pyrrolidin-1-ylprop-1-enyl]pyridine”, the IUPAC name.
To understand the above statements one need to be familiar with the “cis-trans and E-/Z- nomenclature. How to translate the name to structure and vice versa? This blog is basically to discuss the “cis-trans and E-/Z- notations employed while naming organic molecules and enable to appreciate the nomenclature system with live examples from pharmaceuticals.
When two atoms are connected by a single bond the barrier to free rotation about this bond is quite small. On the contrary, when two atoms are linked by multiple bonds then the barrier to rotation is much higher and where such compounds exist in two forms the passage from one to the other requires so much energy that each isomer has a stable and independent existence. In other words, free rotation cannot happen across a carbon to carbon double bond without disruption of the bond. Cis-trans isomers do not rotate plane polarized light (unless they also contain a stereogenic center) and hence are not chiral. Other structural attribute that can offer barrier to rotation are cyclic and allene systems. The post focuses on the double bonded carbon-carbon and carbon-heteroatom systems.
Cis-trans Isomerism due to Double bonded carbon-carbon systems
The term cis-trans isomerism indicates the type of diastereomer that occurs as a result of restricted rotation around a bond, as in olefinic compounds. This is sometimes also referred to as π-diastereoisomerism. This term is more useful than the usual designations of cis-trans or geometrical isomerism since it conveys the chemical origin and the correct description of the stereoisomerism. It also avoids any confusion with cis-trans isomerism in cyclic systems where no double bond is involved.
Nomenclature across double bonds
Cis-trans notation
Diastereoisomerism about double bond is more common and are usually designated as cis-trans isomerism. If the alkene is designated as abC=Ccd then it will exist in two forms when a ≠ b and c ≠ d. Note that (I) and (II) are diastereomers. There are two ways to name such isomers. In the older system, one isomer is called cis- and the other trans-. In (I) and (II) when a = c, results in the cis- isomer (III) and the trans– isomer (IV) respectively.
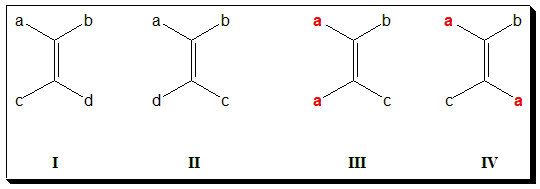
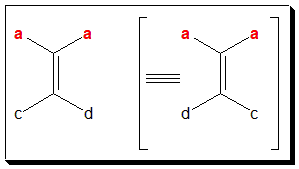
In (I)/(II), if either a=b or c=d, the molecule will not exhibit geometric isomerism. In (V), you find a = b and hence will not exhibit cis-trans isomerism.
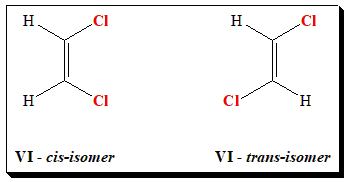
Let us take the case of 1,2-dichloroethene to illustrate the above notations. In (VI), the two chlorine atoms are locked on the same side of the double bond. This is known as the cis isomer. (cis: from latin meaning “on this side”). In (VII), the two chlorine atoms are locked on opposite sides of the double bond. This is known as the trans isomer. (trans: from latin meaning “across” – as in transatlantic).
E-Z notation: when cis-trans notation cannot be used
When the four groups attached to the double bond of a molecule are different, it is not convenient to use cis-trans nomenclature. The best and unambiguous way of notating two such diastereomers is the (E-Z) system, based on priorities of groups in the Cahn-Ingold-Prelog sequence rule.
- Apply sequence rules to assign priorities to the two groups attached to each doubly bonded carbon atoms
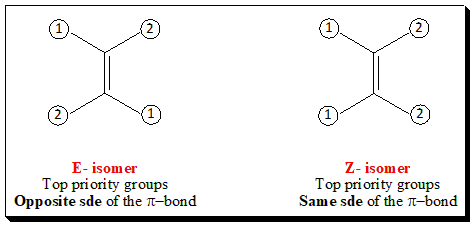
- If the two groups of highest priority groups are on opposite sides of the double bond, the isomer is designated E (from the German word Entgegen meaning oppositeor across); When the highest priority groups are on the same side of the double bond, the isomer is designated Z (from the German word Zusammen meaning together); Note: A useful mnemonic here is that zusammen contains the word ‘same’.
The E-Z system of nomenclature is illustrated below. That isomer which has the two highest ranking groups, based on sequence rules, on the same side of the double bond is called (Z) and other isomer is designated (E).
Live example
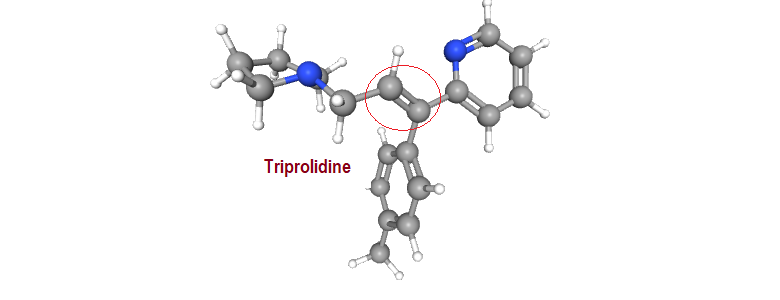
A typical example of a drug molecule that exhibits geometrical isomerism is the antihistaminic agent triprolidine. The configuration is designated using the E-Z notation.
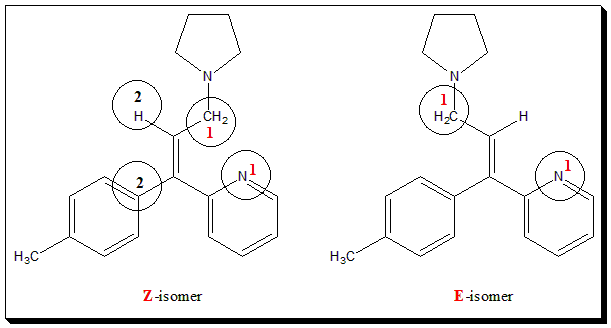
Self test: Comment on the structural influences (geometrical isomers) on the antihistaminic activity
Other examples from pharmaceuticals that demonstrate geometrical isomerism include the antidepressant doxepin, the nonsteroidal synthetic estrogen diethylstilbestrol, antiestrogen tamoxifen, antihistaminic triprolidine and the neuroleptic thiotixene.
Practice exercise
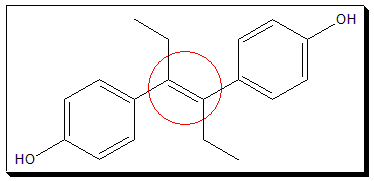
Assign cis-/trans- or E-Z notation, as appropriate, to the the following drug molecules (i and ii).
I. Diethylstilbestrol, a synthetic nonsteroidal estrogen helps to control the symptoms of some cancers.
ii. Doxepin, the tricyclic antidepressant, is a medication used to treat major depressive disorder, anxiety disorders, chronic hives, and trouble sleeping.
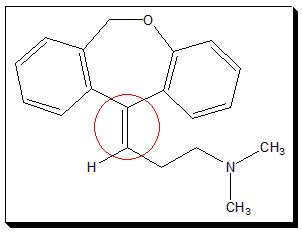
Cis-trans Isomerism due to Double bonded carbon-heteroatom systems
So far we have confined our discussion of π-diastereoisomerism to C=C system. But one or both carbon atoms can be replaced by another doubly bonded atom. Most commonly this is nitrogen in which the sp2 orbital contains only a lone pair of electron. For example, oximes and azo compounds exhibit cis-trans-isomerism and exist in stereoisomeric forms. In the past special prefixes (syn and anti) have been used to designate these isomers. In these cases the priority assignments are made as described previously, and the isomer with the highest priorities on the same face of the double bond are called syn, whereas those with these groups on opposite sides are named anti.
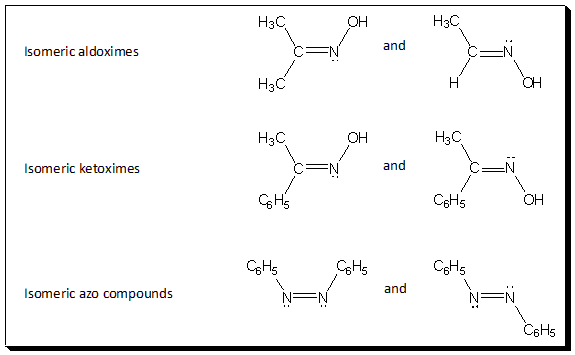
Self test: Designate the above six structures as (E) or (Z).
Further reading
Jerry March, Advanced Organic Chemistry: Reactions, Mechanisms and Structure, John Wiley & Sons Inc., New York, 2006.
Wilson and Gisvold’s Textbook of organic medicinal and pharmaceutical chemistry, Edited by John H. Block and John M. Beale, Jr., 12th edition, Lippincott Williams & Wilkins, New York, 2010.
Foye’s Principles of Medicinal Chemistry, Lemke, Thomas L. (Editor), Williams, David A. (Editor), Roche, Victoria F. (Editor), 7th edition, Lippincott Williams & Wilkins, New York, 2012.
Bernard Testa, Principles of organic stereochemistry, Marcel Dekker Inc., New York, 1979.

Great! Clearly explained about E-Z isomers. I raise some points. if possible please clarify.
As I know Z to E isomerization is much more feasible than vice versa? Is there any report or possibility for E-Z isomerization? If, how would be?
“Pharmacological studies confirm the high activity of triprolidine and the superiority of (E) over corresponding (Z) isomers as H, antagonist” – Is there any Z isomer molecule that has high activity than the E isomer?
Thanks for your comments. Regarding E-Z isomerization, number of article ae available on reversible Z_E isomerization. You may go through that. For the other point, there ae examples where the Z-isomer is used. Tamoxifen, the anticancer agent, is an example where the Z-isomer is available as the product. Whether the E- or Z- isomer is active depends on the position of the pharmacophoric groups and their interaction with the complimentary sites on the receptor.
Good morning Sir
Excellent explanation of isomers
Please highlight about SAR and isomers Sir
G Krishnamoorthy Periyar College of Pharmaceutical Sciences Trichy
Sir could you please highlight on significance of oxime chemistry in cephalosporins, thank you
Sure. I shall post a blog on the E/Z and syn/anti system of nomenclature applied to cephalosporins in due course of time.
Kindly add few more topics like Racemic modification and resolution of racemic mixture…some will find useful on that also..
Good work sir…
Definitely I shall write on the topics you have asked for in due course of time. Keep tracking chiralpedia.com. Meanwhile you can go through my contribution in Wikipedia on chiral separations @ the following link <https://en.wikipedia.org/wiki/Chiral_analysis>.
I have read cis trans and E-Z , meso componds, D / L , it is useful for me and I have content about absolute configuration.
Thanks you find it useful.
Please cover the recmic modification topics with situable examples
Definitely I shall write on the topic you have asked for in due course of time.