Synopsis
Welcome to an exploration of Chiral Switch!!! Chiral switching revolutionizes the pharmaceutical landscape by transforming racemic drugs into their more effective single-enantiomer forms, enhancing therapeutic efficacy and reducing side effects This blog post delves into the intriguing realm of chiral switches, presenting key concepts such as chiral switch strategies, their advantages, and the regulatory environment focusing on bridging studies. An engaging mind map highlights notable case studies, both successful and unsuccessful, and also examines Evergreening strategies and Metabolite switches. Discover how single enantiomers are poised to revolutionize the pharmaceutical landscape and unlock new therapeutic potentials. <For more expert insights and a detailed exploration of this fascinating topic, don’t miss the “Further Reading” section>.
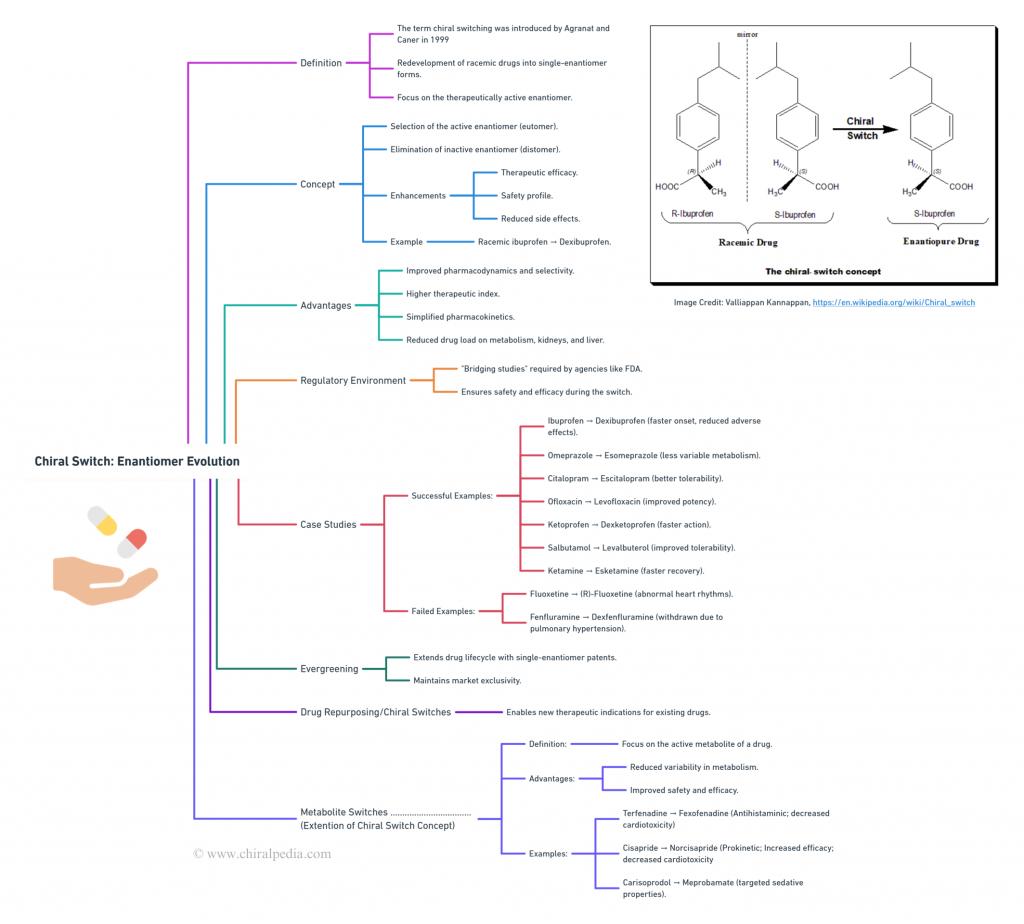
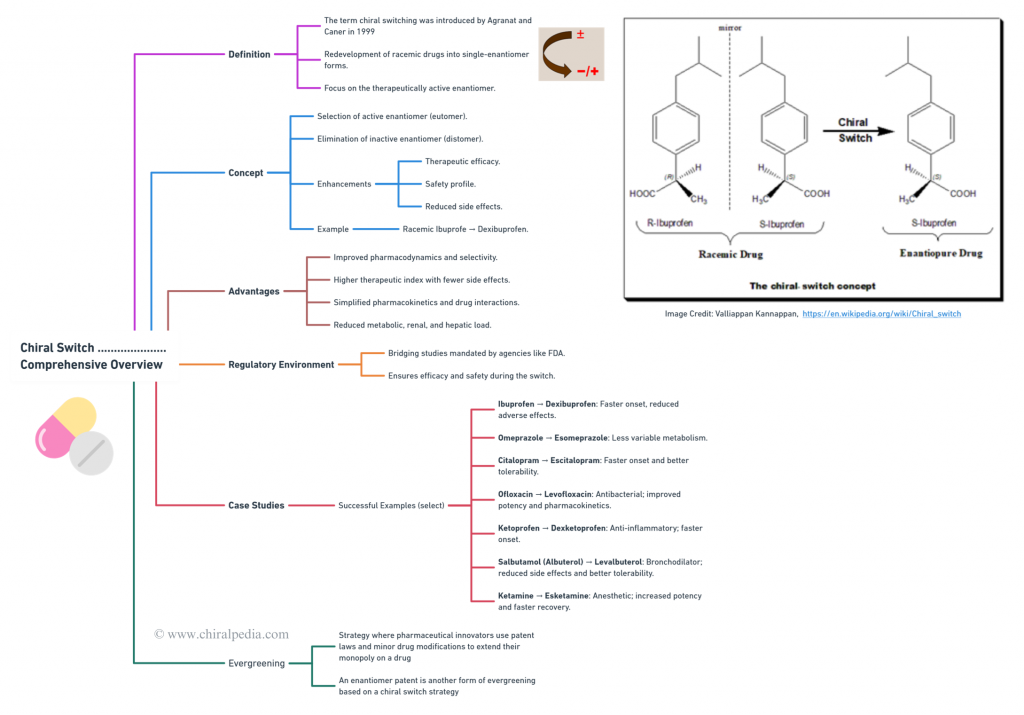
(Core mind map)
Regulatory agencies like the FDA ensure safe and effective transitions from racemic drugs to single-enantiomer versions through rigorous bridging studies. Case-studies encompass success stories like ibuprofen to dexibuprofen, omeprazole to esomeprazole, and citalopram to escitalopram showcase the benefits of chiral switches, including faster onset and reduced side effects. However, some switches face safety issues, such as (R)-Fluoxetine and dexfenfluramine. Evergreening strategies extend the lifecycle of drugs by introducing patentable single-enantiomer versions, enabling new therapeutic applications and highlighting the innovative potential of chiral chemistry. Additionally, Metabolite switches focus on redeveloping drugs based on their active metabolites, further expanding the scope and impact of the Chiral Switch approach. 🌟
Chiral Switch: Comprehensive Overview
Introduces readers to the captivating world of chiral switches. Utilizing an engaging mind map, this blog explores key concepts like the chiral switch strategy, its advantages, the regulatory environment emphasizing bridging studies, and notable case studies (both successes and failures). It also delves into Evergreening strategies, showcasing the potential of single enantiomers to revolutionize the pharmaceutical landscape. 🌟🧬✨
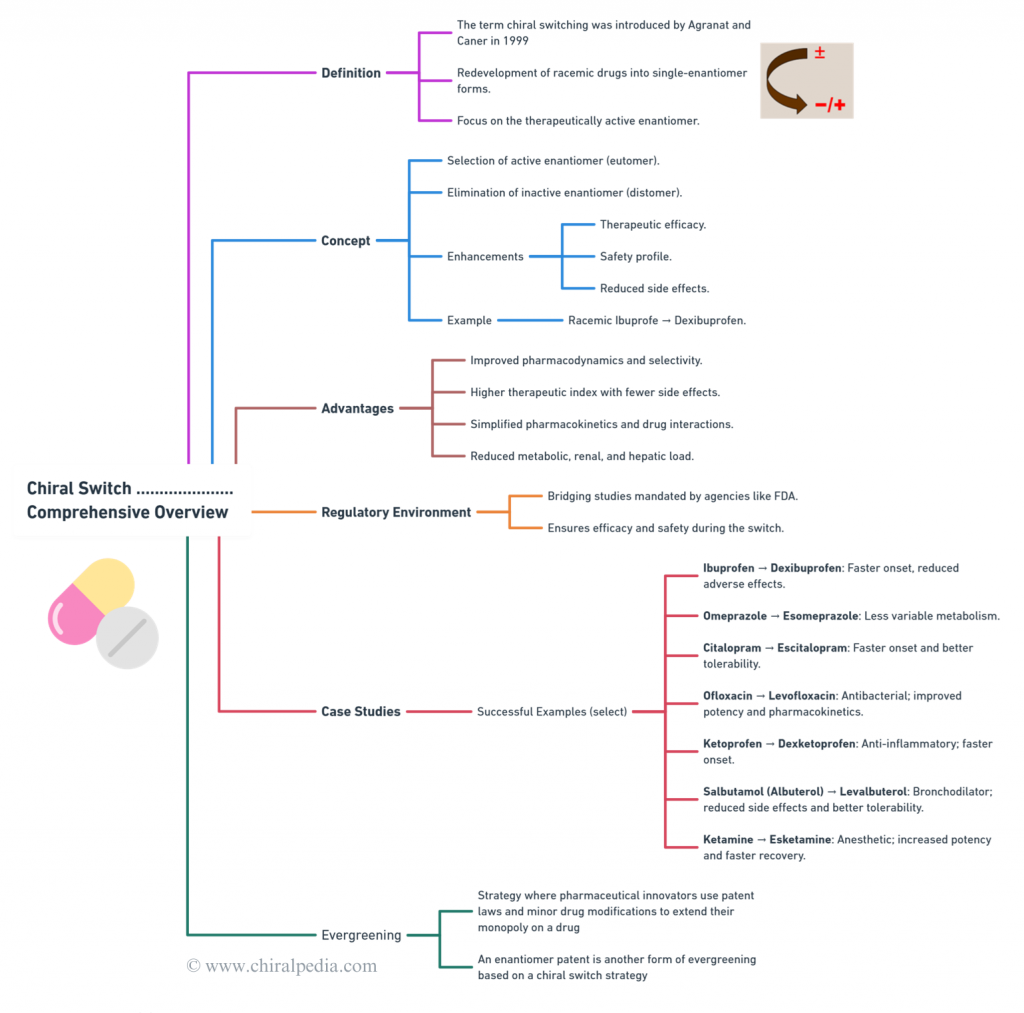
(focus being on Chiral Switch Strategy)
Conclusion
The Chiral Switch strategy has already demonstrated its potential to enhance therapeutic outcomes and safety profiles for multiple drugs. By transitioning from racemic drugs to their single-enantiomer forms, substantial improvements in pharmacodynamics, therapeutic index, and drug interactions have been achieved. The strategy also supports evergreening, extending the lifecycle and market exclusivity of pharmaceutical products
Future Direction
Looking ahead, the focus on Chiral Switches is likely to grow, driven by advancements in analytical techniques and regulatory frameworks. Future research may explore:
- Discovery of Novel Single-Enantiomer Drugs: Identifying and developing new single-enantiomer drugs from existing racemates to optimize therapeutic benefits.
- Enhanced Analytical Methods: Improving techniques to precisely assess chiral purity and enantiomer-specific effects, ensuring the highest standards.
- Broader Applications: Expanding the use of Chiral Switches to drugs across different therapeutic categories, including those with complex metabolic profiles.
- Environmental Sustainability: Developing environmentally friendly processes for the synthesis and production of single-enantiomer drugs.
- Personalized Medicine: Integrating Chiral Switch strategies with personalized medicine approaches to tailor therapies to individual patient profiles for optimal outcomes.
By embracing these advancements, the Chiral Switch strategy can continue to unlock the full potential of single-enantiomer drugs, ultimately enhancing patient care and advancing pharmaceutical science.
Further Reading
Agranat I, Wainschtein SR (March 2010). “The strategy of enantiomer patents of drugs”. Drug Discovery Today. 15 (5–6): 163–170. doi:10.1016/j.drudis.2010.01.007. PMID 20116449.
Caner H, Groner E, Levy L, Agranat I (February 2004). “Trends in the development of chiral drugs”. Drug Discovery Today. 9 (3): 105–110. doi:10.1016/s1359-6446(03)02904-0. PMID 15038394.
Agranat I, Caner H (July 1999). “Intellectual property and chirality of drugs”. Drug Discovery Today. 4 (7): 313–321. doi:10.1016/s1359-6446(99)01363-x. PMID 10377509.
Agranat I, Caner H, Caldwell J (October 2002). “Putting chirality to work: the strategy of chiral switches”. Nature Reviews. Drug Discovery. 1 (10): 753–768. doi:10.1038/nrd915. PMID 12360254. S2CID 1543301.
Ariëns EJ (1984). “Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology”. European Journal of Clinical Pharmacology. 26 (6): 663–668. doi:10.1007/bf00541922. PMID 6092093. S2CID 30916093.
Ariëns EJ (1986). “Stereochemistry: a source of problems in medicinal chemistry”. Medicinal Research Reviews. 6 (4): 451–466. doi:10.1002/med.2610060404. PMID 3534485. S2CID 36115871.
Ariëns EJ, Wuis EW, Veringa EJ (January 1988). “Stereoselectivity of bioactive xenobiotics. A pre-Pasteur attitude in medicinal chemistry, pharmacokinetics and clinical pharmacology”. Biochemical Pharmacology. 37 (1): 9–18. doi:10.1016/0006-2952(88)90749-PMID 3276322.
Ariëns EJ (1991). “Racemic therapeutics–ethical and regulatory aspects”. European Journal of Clinical Pharmacology. 41 (2): 89–93. doi:10.1007/BF00265897. PMID 1743252. S2CID 12768116.
Jamali F, Mehvar R, Pasutto FM (September 1989). “Enantioselective aspects of drug action and disposition: therapeutic pitfalls”. Journal of Pharmaceutical Sciences. 78 (9): 695–715. doi:10.1002/jps.2600780902. PMID 2685226.
Drayer DE (August 1986). “Pharmacodynamic and pharmacokinetic differences between drug enantiomers in humans: an overview”. Clinical Pharmacology and Therapeutics. 40 (2): 125–133. doi:10.1038/clpt.1986.150. PMID 3731675. S2CID 33537650.
Ariens EJ (1989). Krstulovic AM (ed.). Chiral Separations by HPLC. Ellis Horwwod, Chichester. pp. 31–68.
Cayen MN (1991). “Racemic mixtures and single stereoisomers: Industrial concerns and issues in drug development”. Chirality. 3 (2): 94–98. doi:10.1002/chir.530030203. ISSN 0899-0042.
Evans AM, Nation RL, Sansom LN, Bochner F, Somogyi AA (December 1988). “Stereoselective drug disposition: potential for misinterpretation of drug disposition data”. British Journal of Clinical Pharmacology. 26 (6): 771–780. doi:10.1111/j.1365-2125.1988.tb05318.x. PMC 1386594. PMID 3242583.
Walle T, Walle UK (1986). “Pharmacokinetic parameters obtained with racemates”. Trends in Pharmacological Sciences. 7: 155–158. doi:10.1016/0165-6147(86)90294-4. ISSN 0165-6147.
Gross M, Cartwright A, Campbell B, Bolton R, Holmes K, Kirkland K, et al. (1993). “Regulatory Requirements for Chiral Drugs”. Drug Information Journal. 27 (2): 453–457. doi:10.1177/009286159302700232. ISSN 0092-8615. S2CID 72629140.
Nguyen LA, He H, Pham-Huy C (June 2006). “Chiral drugs: an overview”. International Journal of Biomedical Science. 2 (2): 85–100. doi:10.59566/IJBS.2006.2085. PMC 3614593. PMID 23674971.
Aboul-Enein HY, Wainer IW (1997). The impact of stereochemistry on drug development and use. New York: Wiley. ISBN 0-471-59644-2. OCLC 35262289.
Tucker GT (March 2000). “Chiral switches”. Lancet. 355 (9209): 1085–1087. doi:10.1016/s0140-6736(00)02047-x. PMID 10744105. S2CID 30715334.
Mansfield P, Henry D, Tonkin A (2004). “Single-enantiomer drugs: elegant science, disappointing effects”. Clinical Pharmacokinetics. 43 (5): 287–290. doi:10.2165/00003088-200443050-00002. PMID 15080762. S2CID 31664339.
Tomaszewski J, Rumore MM (1994). “Stereoisomeric Drugs: FDA’S Policy Statement and the Impact on Drug Development”. Drug Development and Industrial Pharmacy. 20 (2): 119–139. doi:10.3109/03639049409039080. ISSN 0363-9045.
Gross M (1991). “Development of chiral drug in an evolving regulatory environment”. Regulatory Affairs. 3: 483–494.
Stinson SC (1993-09-27). “CHIRAL DRUGS”. Chemical & Engineering News Archive. 71 (39): 38–65. doi:10.1021/cen-v071n039.p038. ISSN 0009-2347.
Stinson SC (1995-10-09). “CHIRAL DRUGS”. Chemical & Engineering News Archive. 73 (41): 44–546274. doi:10.1021/cen-v073n041.p044. ISSN 0009-2347.
Kumkumian CS (1990). “Regulatory Considerations concerning Stereoisomers in Drug Products”. Drug Information Journal. 24 (1): 125–127. doi:10.1177/009286159002400124. ISSN 0092-8615. S2CID 72604547.
Calcaterra A, D’Acquarica I (January 2018). “The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds”. Journal of Pharmaceutical and Biomedical Analysis. 147: 323–340. doi:10.1016/j.jpba.2017.07.008. PMID 28942107. S2CID 6922311.
Hancu G, Modroiu A (February 2022). “Chiral Switch: Between Therapeutical Benefit and Marketing Strategy”. Pharmaceuticals. 15 (2): 240. doi:10.3390/ph15020240. PMC 8877306. PMID 35215352.
Hancu G, Modroiu A (February 2022). “Chiral Switch: Between Therapeutical Benefit and Marketing Strategy”. Pharmaceuticals. 15 (2): 240. doi:10.3390/ph15020240. PMC 8877306. PMID 35215352.
Gaudry KS (October 2011). “Evergreening: a common practice to protect new drugs”. Nature Biotechnology. 29 (10): 876–878. doi:10.1038/nbt.1993. PMID 21997625. S2CID 19402161.
Somogyi A, Bochner F, Foster D (2004). “Inside the isomers: the tale of chiral switches”. Australian Prescriber. 27 (2): 47–49. doi:10.18773/austprescr.2004.039. hdl:2440/39339.
D’Acquarica I, Agranat I (2023-01-17). “The Quest for Secondary Pharmaceuticals: Drug Repurposing/Chiral-Switches Combination Strategy”. ACS Pharmacology & Translational Science. 6 (2): 201–219. doi:10.1021/acsptsci.2c00151. ISSN 2575-9108. PMC 9926527. PMID 36798472.
https://en.wikipedia.org/wiki/Chiral_switch