Introduction
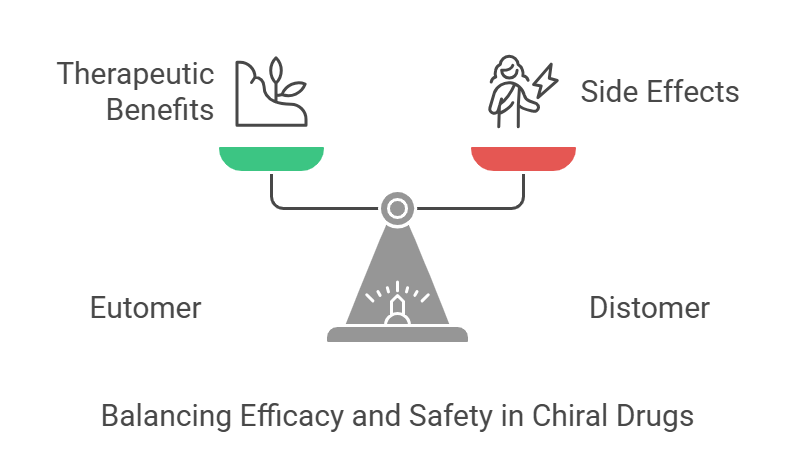
Chirality, or “handedness,” is the unique property of molecules that exist as non-superimposable mirror images, known as enantiomers. Drugs that exhibit handedness are referred to as chiral drugs. Chiral medications consisting of an equal (1:1) mixture of enantiomers are called Racemic drugs. In drug development, chirality is incredibly important because most biological molecules, like enzymes and receptors, are chiral. This characteristic affects how a drug interacts with biological targets, leading to significant differences in how enantiomers behave in the body (Ceramella et al., 2022; González-Rojano et al., 2020). For example, the “eutomer” (the more active enantiomer) can provide therapeutic benefits, while the “distomer” (the less active enantiomer) might cause side effects or toxicity (Mehvar and Jamali, 1997; Lees et al., 2012; https://en.wikipedia.org/wiki/Chiral_drugs).
Chiral bioequivalence studies are all about understanding how drugs that exist as enantiomers – mirror-image molecules -behave in our bodies. These studies are super important for grasping the different pharmacokinetics and pharmacodynamics of each enantiomer. Since enantiomers can have markedly different effects, it’s essential to evaluate them individually in bioequivalence studies, especially when their pharmacological activities vary.
Need and Importance
The need for chiral bioequivalence studies has become increasingly important as chiral drugs play a vital role in pharmacotherapy. Conducting these studies is crucial to ensure patient safety and therapeutic efficacy. Many drugs are sold as racemates (a mix of both enantiomers), but often only one enantiomer is responsible for the therapeutic effect. Take ibuprofen, for example -the S-enantiomer is mainly responsible for its anti-inflammatory properties, while the R-enantiomer is less effective and undergoes chiral inversion (González-Rojano et al., 2020; Juan José Torrado et al., 2010). Even slight differences in the pharmacokinetics of enantiomers – such as absorption rates, metabolic pathways, or protein binding – can alter therapeutic outcomes, emphasizing the necessity of stereospecific bioequivalence studies (Reig-López et al., 2024; Lees et al., 2012; García-Arieta and Gordon, 2012).
Regulatory bodies like the FDA and EMA recommend measuring individual enantiomers when they exhibit different pharmacokinetic or pharmacodynamic properties, especially if one enantiomer is primarily responsible for the therapeutic effects (Juan José Torrado et al., 2010; Mehvar R and Jamali F, 1997). Similarly, EMA guidelines emphasize chiral bioequivalence assessment for enantiomers with unique clinical or safety profiles (Lees et al., 2012).
Relevance of Chiral Bioequivalence
Several case studies illustrate the relevance of chiral bioequivalence. These examples underscore the critical role that chiral bioequivalence studies play in ensuring that patients receive safe and effective medications tailored to their specific needs.
Ibuprofen:
Studies have highlighted cases where non-stereospecific methods failed to detect critical bioequivalence issues. For example, a bioequivalence study of ibuprofen formulations using non-stereospecific methods concluded equivalence, but stereospecific assays revealed significant differences in S-enantiomer profiles (Torrado et al., 2010).
| Method | Parameter Assessed | Outcome |
| Non-Stereospecific | Total ibuprofen | Equivalence observed but discrepancies in enantiomer profiles overlooked |
| Stereospecific | S-enantiomer PK | Differences in absorption and metabolism identified, ensuring accurate therapeutic assessment |
Carvediol:
Similar findings were reported for carvedilol, where stereospecific techniques identified variations in enantiomer activity that would have gone unnoticed with achiral methods (González-Rojano et al., 2020).
| Method | Parameter Assessed | Outcome |
| Non-Stereospecific | Total carvedilol | Variability in enantiomer contributions masked |
| Stereospecific | Enantiomer-specific PK | Differences in β-blocking and antioxidant activity identified, improving therapeutic equivalence |
Racemic Drugs:
A comprehensive study of various racemic drugs found that for drugs with nonlinear pharmacokinetics or significant differences in the effects of enantiomers, measuring the total drug could lead to inaccurate bioequivalence results. The study suggested guidelines for using stereospecific assays when enantiomers show major differences in kinetic parameters or convert to their mirror image during metabolism. This recommendation was backed by both simulations and experimental data, showing that stereospecific evaluations provide a more accurate reflection of therapeutic outcomes (Mehvar and Jamali, 1997).
Bioequivalence Reports: Comparing conventional and stereospecific methods
Various case studies have delved into comparing conventional and stereospecific methods in bioequivalence assessments. To make the findings from these studies on chiral drugs -like ibuprofen, flurbiprofen, disopyramide, and verapamil—easier to grasp, we’ve highlighted the key points in a mind map
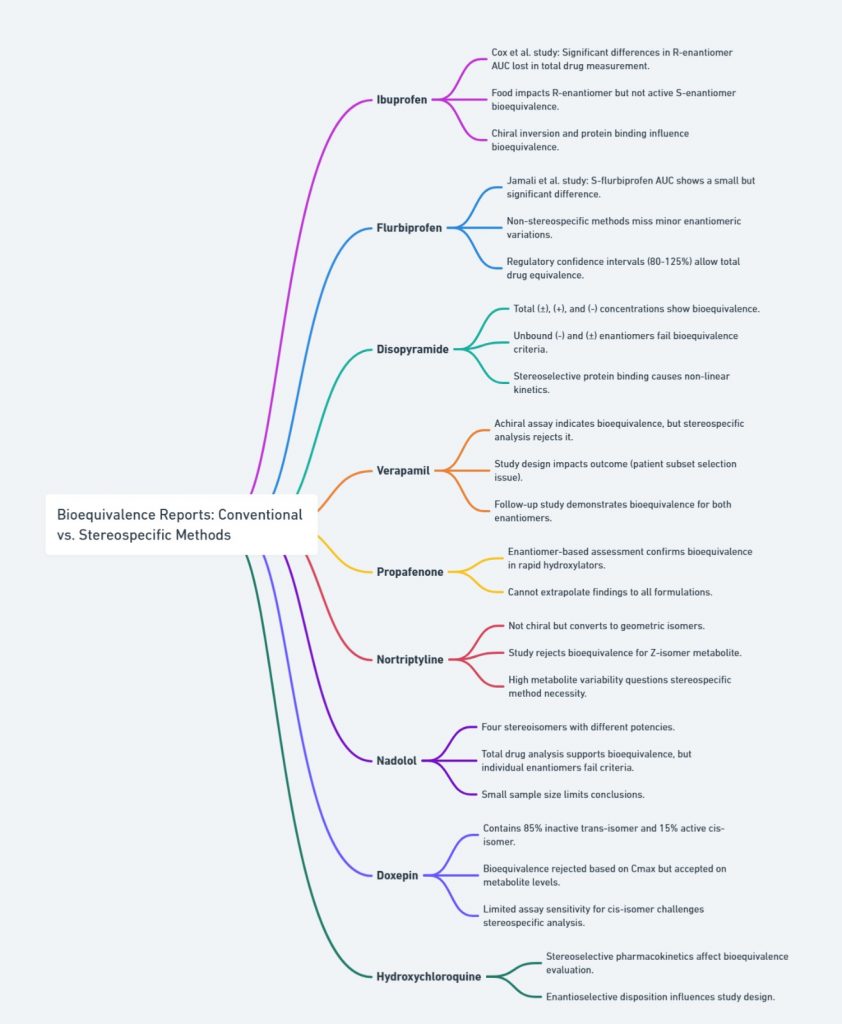
Several case studies have examined how conventional and stereospecific methods compare in bioequivalence assessments. Some drugs show no difference between the two approaches, while others demonstrate significant variations when individual enantiomers are analyzed. This figure organizes key findings from bioequivalence studies of multiple chiral drugs, including ibuprofen, flurbiprofen, disopyramide, verapamil, and more.
Design of Bioequivalence Studies of Chiral Drugs
When designing bioequivalence (BE) studies for chiral drugs, several key factors come into play: drug characteristics, formulation factors, statistical considerations, and the stereoselective analytical method. Each of these aspects significantly impacts the study, and their effects are presented through flow charts to make the information easier to understand. For a deeper dive into the topic, check out the further reading section (Mehvar and Jamali, 1997).
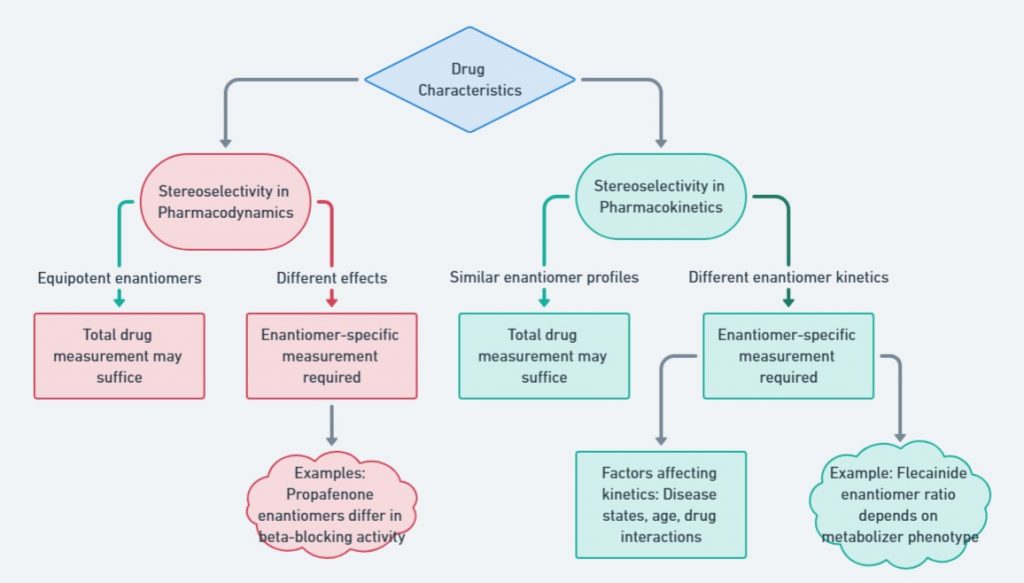
Stereoselectivity plays a critical role in drug bioequivalence, influencing both pharmacodynamics and pharmacokinetics. While some enantiomers exhibit identical therapeutic effects, others may have significantly different potencies or adverse effects. This figure summarizes the decision process for determining when enantiomer-specific measurements are necessary.
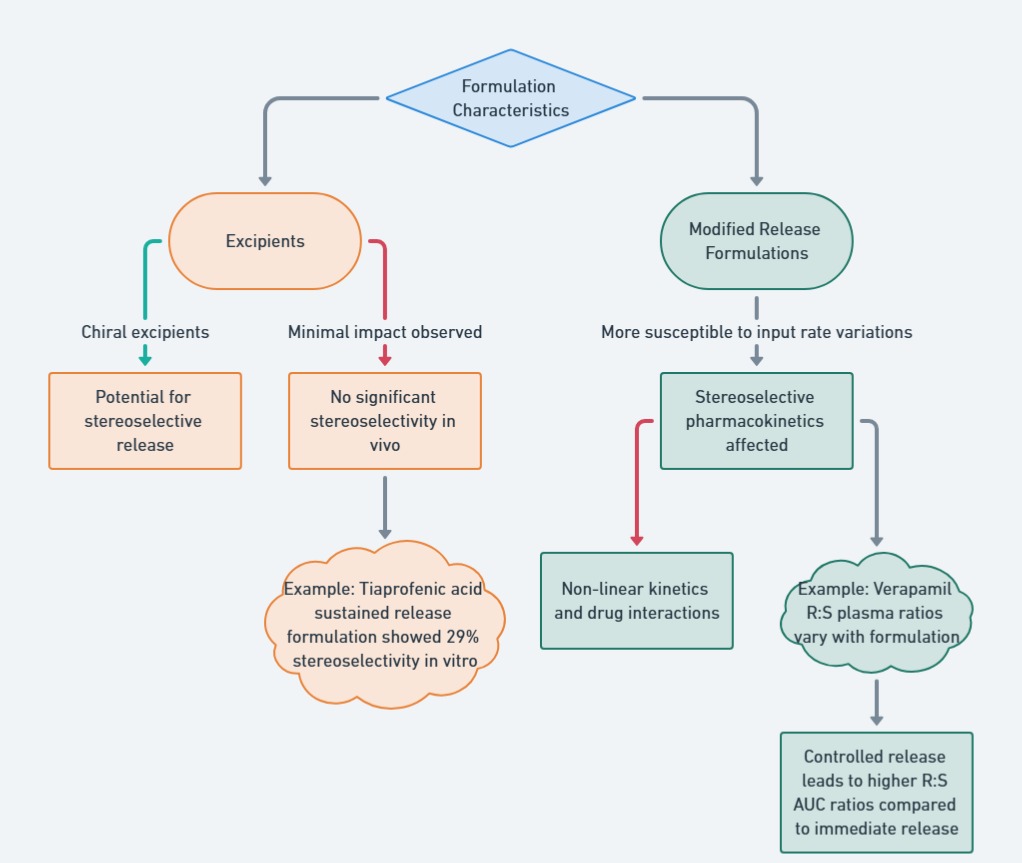
The choice of formulation can impact the stereoselectivity of a drug. Certain excipients and modified-release formulations may alter enantiomeric ratios in ways that affect bioavailability. This figure highlights cases where stereospecific analysis may be required due to formulation-related factors.
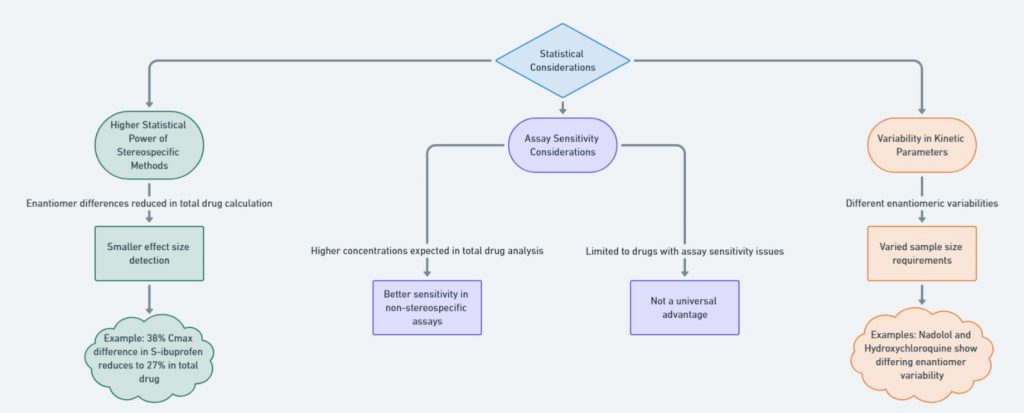
Stereospecific bioequivalence studies offer higher statistical power by isolating enantiomeric differences that might otherwise be diluted in total drug measurements. This figure discusses how statistical variability and assay sensitivity influence bioequivalence outcomes.
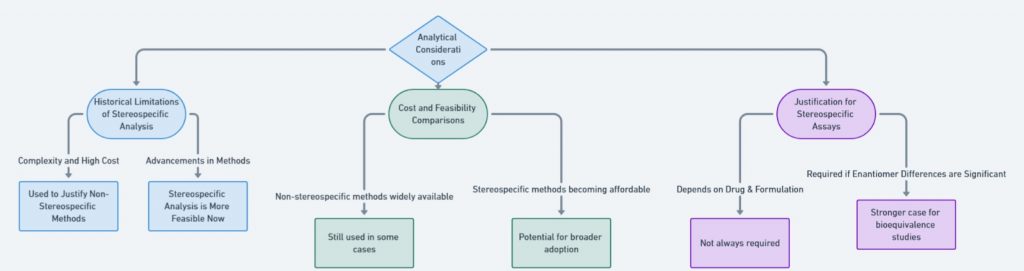
Advancements in analytical methods have made stereospecific assays more feasible. However, the choice between stereospecific and non-stereospecific methods depends on the drug’s characteristics. This figure contrasts the cost, feasibility, and necessity of stereospecific assays.
Chiral Bioanalytical Tools
Chiral methods are tailored to measure and analyze each enantiomer of chiral compounds individually. They become especially important when the enantiomers have different pharmacological effects or pharmacokinetic properties, as these differences can greatly influence the drug’s effectiveness and safety (Juan José Torrado et al., 2010; <https://en.wikipedia.org/wiki/Chiral_analysis>)
Some common techniques for analyzing enantiomers include chiral chromatography (like HPLC with chiral stationary phases), chiral capillary electrophoresis, and NMR spectroscopy with chiral shift reagents. These methods take advantage of the unique interactions between enantiomers and chiral selectors to achieve separation. They can detect subtle differences in pharmacokinetics, like variations in absorption rates (Cmax) and exposure (AUC) between enantiomers. These details are crucial for proving bioequivalence in regulatory submissions. Regulatory agencies often require chiral analysis for drugs where enantiomers have markedly different effects or safety profiles. This is particularly true for drugs where one enantiomer is therapeutically active while the other is not or may even be harmful.
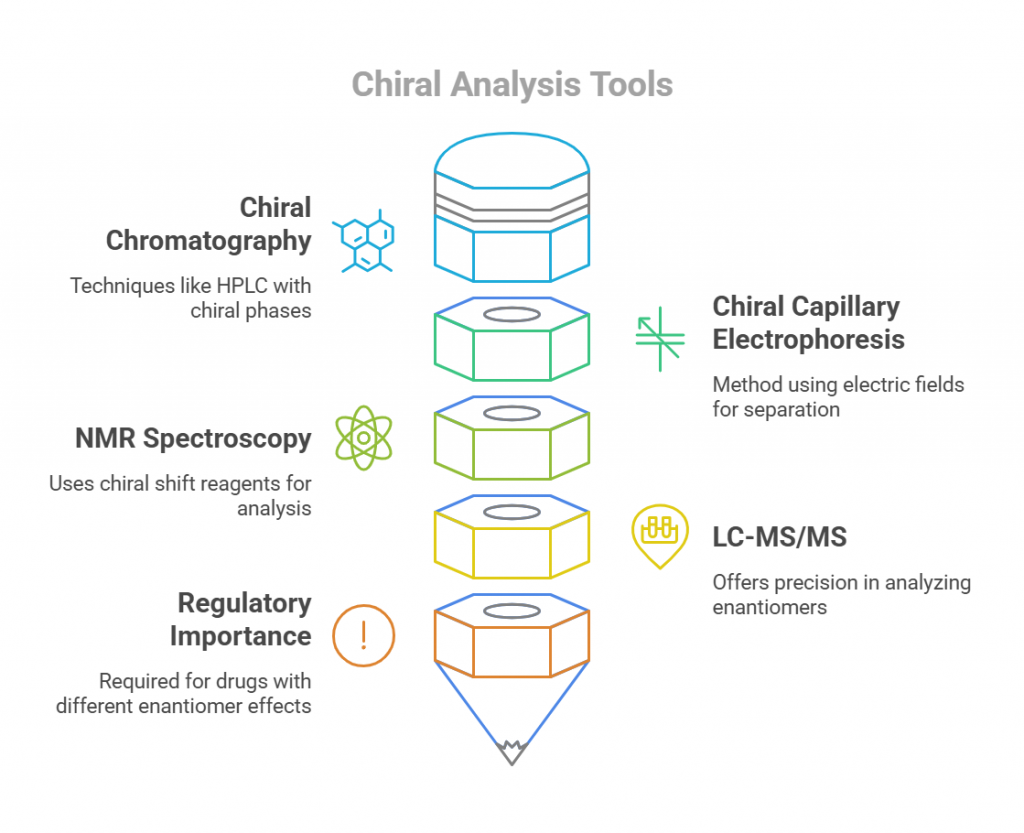
Some common techniques for analyzing enantiomers include chiral chromatography (like HPLC with chiral stationary phases), chiral capillary electrophoresis, and NMR spectroscopy with chiral shift reagents. These methods take advantage of the unique interactions between enantiomers and chiral selectors to achieve separation. They can detect subtle differences in pharmacokinetics, like variations in absorption rates (Cmax) and exposure (AUC) between enantiomers. These details are crucial for proving bioequivalence in regulatory submissions. Regulatory agencies often require chiral analysis for drugs where enantiomers have markedly different effects or safety profiles. This is particularly true for drugs where one enantiomer is therapeutically active while the other is not or may even be harmful.
Techniques such as LC-MS/MS and HPLC offer unparalleled precision and sensitivity for analyzing enantiomers. These methods enable the separation and quantification of enantiomers, facilitating a detailed understanding of their individual PK and PD profiles. For instance, LC-MS/MS has been effectively used in ibuprofen studies to assess differences in enantiomer absorption and metabolism (Reig-López et al., 2024). HPLC, with chiral stationary phases, provides robust separation of enantiomers, making it indispensable in regulatory and clinical settings (Ceramella et al., 2022).
Regulatory Challenges and FDA Guidance
The regulatory landscape for chiral drugs reflects the complexity of ensuring safety and efficacy while addressing the unique pharmacokinetic (PK) and pharmacodynamic (PD) profiles of enantiomers. As more chiral drugs enter the market, regulatory agencies, including the FDA and EMA, emphasize the need for enantiomer-specific assessments in drug development and approval processes.
FDA Guidance on Chiral Drug Approval
In 1992, the FDA released crucial guidance on developing chiral drugs, setting key principles for evaluating enantiomers. This guidance emphasizes the need for stereospecific assays when enantiomers show differences in therapeutic activity, pharmacokinetics, or safety profiles. It includes the requirement for stereospecific pharmacokinetic (PK) studies to understand the behavior of individual enantiomers, even in racemic mixtures. The guidance also highlights the importance of assessing chiral inversion, where one enantiomer converts to its mirror image, like with ibuprofen (Jamali and Mehvar, 1997; Reig-López et al., 2024).
Guidelines for Chiral Switching and Labelling
Chiral switching – the process of developing single-enantiomer drugs from racemic mixtures – has become a key regulatory focus. The FDA requires comprehensive evidence showing the superiority of the single enantiomer, including improved efficacy, reduced toxicity, or enhanced PK profiles. Labeling for single-enantiomer drugs must clearly delineate their benefits over racemic counterparts, as seen with escitalopram compared to citalopram (Ceramella et al., 2022).
Regulatory Hurdles in Bioequivalence Studies
Challenges arise when bioequivalence studies rely on non-stereospecific methods, potentially masking clinically significant differences between enantiomers. For example, racemic ibuprofen formulations may appear bioequivalent using total concentration metrics, but stereospecific analysis often reveals discrepancies in the behavior of the active S-enantiomer (García-Arieta et al., 2016). Similarly, in the case of carvedilol, differences in enantiomer-specific activity necessitate stereospecific evaluation to ensure therapeutic consistency across formulations (Torrado et al., 2010).
Harmonization of Global Standards
The lack of standardized international guidelines poses another challenge. While the FDA emphasizes stereospecific assays, the EMA allows for flexibility based on the drug’s PK and PD characteristics. Harmonization of global standards could streamline chiral drug development and approval, reducing costs and regulatory delays. Collaborative efforts between agencies like the International Council for Harmonisation (ICH) aim to address these disparities, promoting consistent requirements for chiral bioequivalence assessments (Lees et al., 2012; Torrado et al., 2010).
Chiral drug development requires navigating a complex regulatory environment. By adopting harmonized standards and leveraging advanced analytical methods, regulatory agencies can better address the challenges associated with enantiomer-specific behavior, ensuring safer and more effective therapies for patients.
Key Insights from Chiral Bioequivalence Case Studies
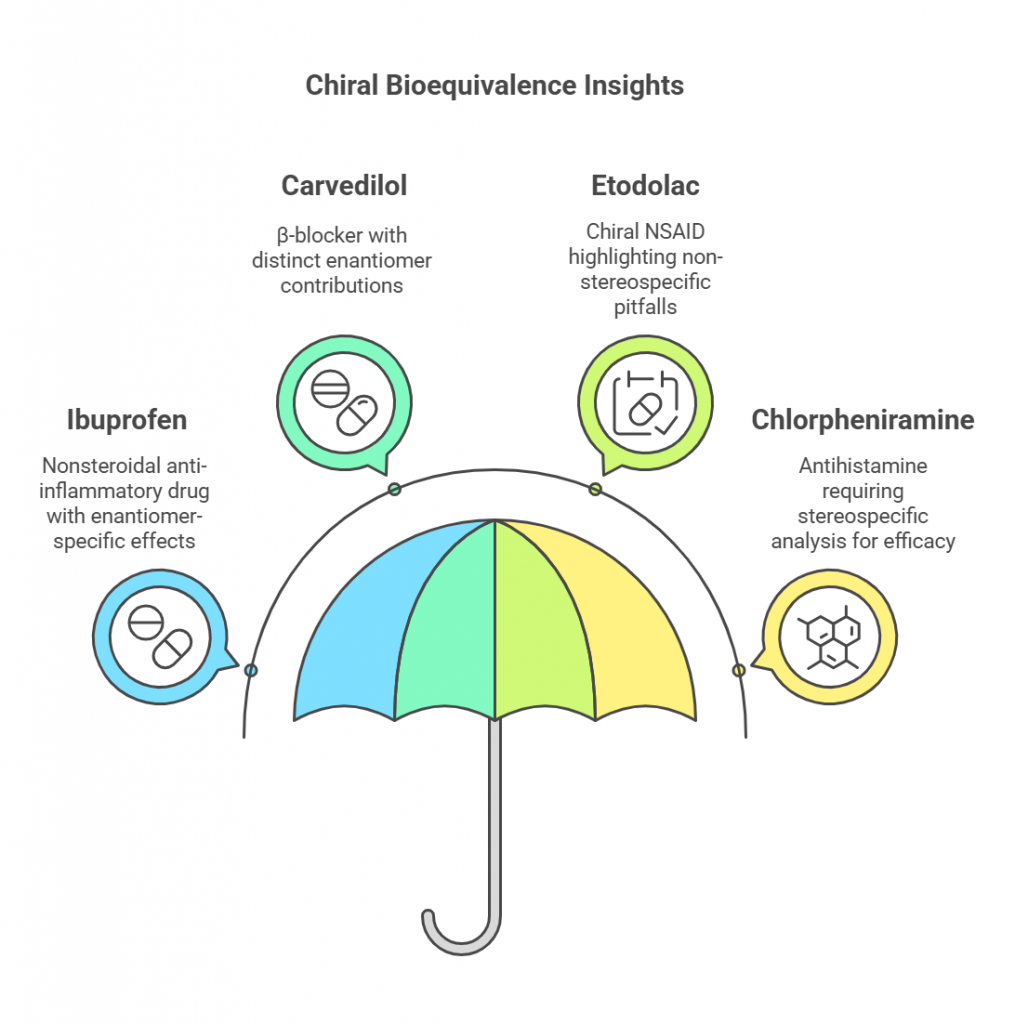
Chiral bioequivalence studies provide valuable insights into the safety, efficacy, and therapeutic equivalence of pharmaceutical formulations containing chiral molecules. By focusing on the pharmacokinetics (PK) and pharmacodynamics (PD) of individual enantiomers, these studies highlight the limitations of non-stereospecific analyses and underscore the clinical relevance of stereospecific assays. This section examines three pivotal case studies – ibuprofen, carvedilol, etodolac, and chlorpheniramine – and discusses their implications for therapeutic outcomes.
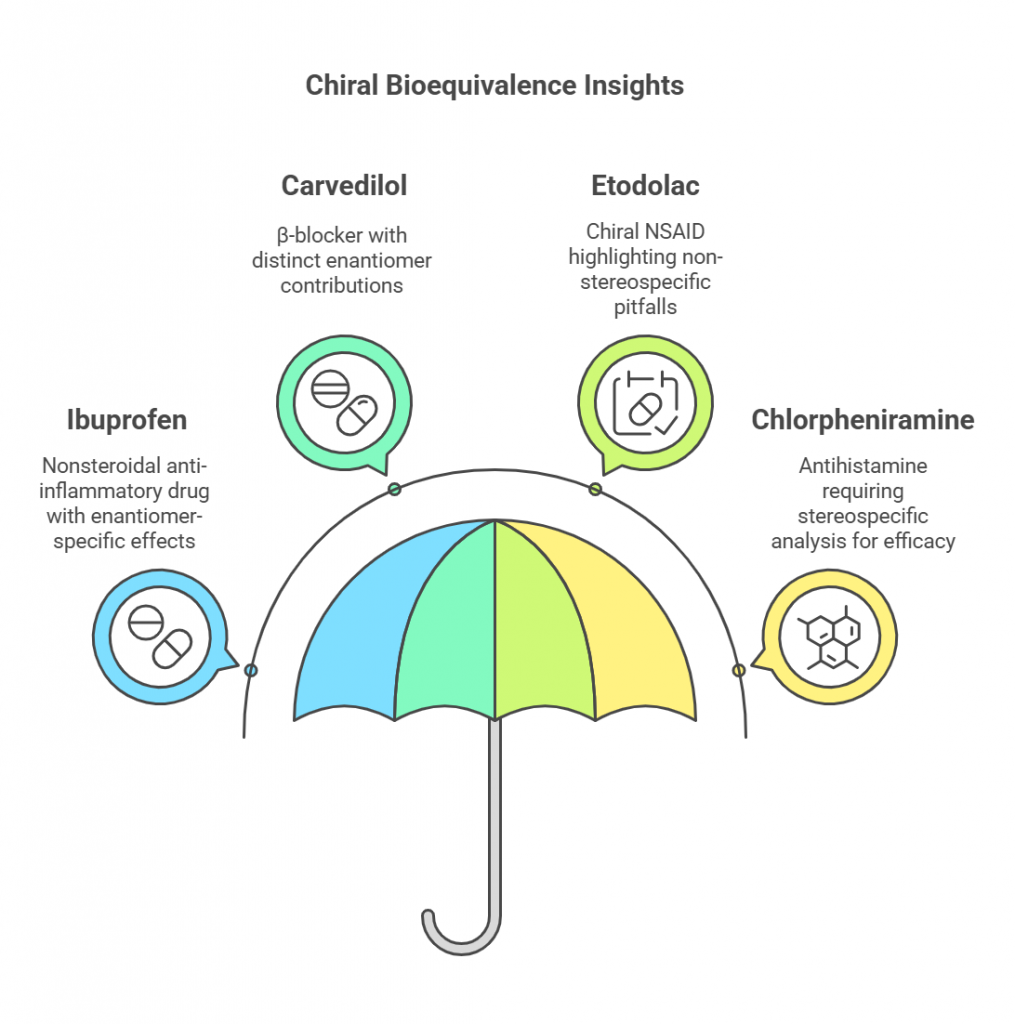
Ibuprofen:
Aa widely used nonsteroidal anti-inflammatory drug (NSAID), is marketed as a racemic mixture of S- and R-enantiomers. The S-enantiomer is the active form, responsible for its anti-inflammatory and analgesic effects, while the R-enantiomer undergoes metabolic conversion to S-ibuprofen in vivo (González-Rojano et al., 2020). Stereospecific bioanalysis has demonstrated significant differences in the PK profiles of these enantiomers, including absorption rates, metabolic pathways, and elimination kinetics.
Studies employing stereospecific assays revealed that non-stereospecific methods often overestimate the therapeutic equivalence of formulations. For instance, formulations appearing bioequivalent based on total ibuprofen concentrations may exhibit disparities in S-enantiomer profiles, impacting efficacy and safety (Reig-López et al., 2024). This highlights the necessity of stereospecific bioanalysis for accurately assessing ibuprofen bioequivalence.
Carvedilol: Challenges with Racemic Mixtures
Carvedilol, a β-blocker used for heart failure and hypertension, is another example where enantiomer-specific analysis is crucial. The S-enantiomer exhibits β-blocking activity, while the R-enantiomer contributes to antioxidant effects (Ceramella et al., 2022). Regulatory challenges arise from the differing pharmacological contributions of these enantiomers in racemic formulations.
Non-stereospecific methods may fail to capture variability in enantiomer PK, particularly when formulations differ in enantiomeric ratios. Stereospecific assays, however, can delineate these differences, improving the accuracy of bioequivalence assessments. Clinical studies have shown that enantiomer-specific analysis ensures consistent therapeutic outcomes across formulations, addressing potential risks associated with racemic bioequivalence evaluations (Torrado et al., 2010).
Etodolac: Demonstrated non-stereospecific criteria could lead to misleading conclusions
In the case of etodolac, a chiral non-steroidal anti-inflammatory drug, research demonstrated that bioequivalence assessments based solely on non-stereospecific criteria could lead to misleading conclusions. The study found that variations in absorption rates significantly influenced the pharmacokinetics of its enantiomers. When comparing two etodolac products, discrepancies in bioavailability were observed when analyzed using chiral methods versus non-chiral methods, highlighting the need for stereospecific assays in evaluating chiral drugs (J R Boni et al., 2000; Mehvar and Jamali, 1997)
Chlorpheniramine: Stereospecific Bioanalysis in Bioequivalence
Chlorpheniramine, an antihistamine, also underscores the importance of stereospecific bioanalysis. Its S-enantiomer is significantly more active than the R-enantiomer, necessitating accurate quantification in bioequivalence studies. Non-stereospecific methods, which assess total drug concentration, fail to differentiate the therapeutic contributions of each enantiomer, potentially leading to misleading conclusions about equivalence (Lees et al., 2012).
Stereospecific methods, such as HPLC with chiral stationary phases, have been instrumental in detecting subtle differences in enantiomer PK and PD profiles. These analyses ensure that generic formulations deliver the intended therapeutic effect without compromising safety, particularly for drugs like chlorpheniramine, where minor variations in enantiomer ratios can significantly impact clinical outcomes (Jamali and Mehvar, 1997).
Clinical Implications of Stereospecific/Chiral Assays
The clinical relevance of stereospecific assays lies in their ability to enhance therapeutic precision. By distinguishing the PK and PD characteristics of individual enantiomers, these methods help optimize dosing regimens, minimize adverse effects, and ensure consistent efficacy across formulations. For example, accurate assessment of S-ibuprofen profiles prevents underdosing, while enantiomer-specific evaluation of carvedilol reduces variability in β-blocker activity.
Bioequivalence Decision for Chiral Drugs
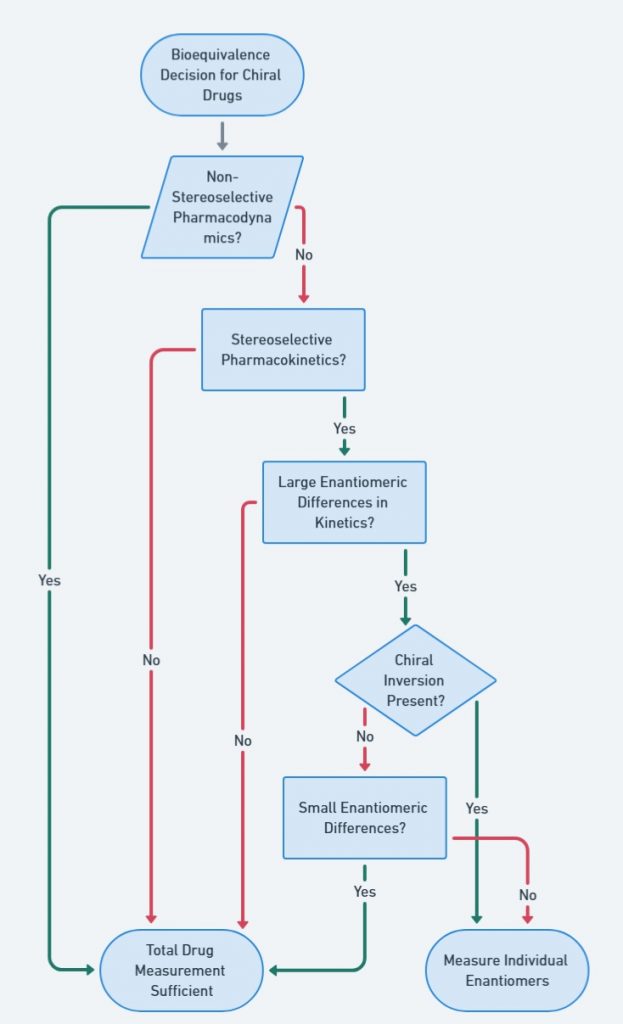
Determining whether a chiral drug requires stereospecific bioequivalence testing depends on multiple factors, including pharmacodynamic differences, enantiomeric kinetics, and chiral inversion. This figure presents a structured decision-making process to assess when enantiomer-specific analysis is justified.
Innovations and Future Directions in Chiral Bioequivalence
Chiral bioequivalence studies are evolving with advancements in technology and methodology, addressing longstanding challenges in enantiomer-specific analysis. Emerging innovations such as AI-driven bioanalysis, machine learning, and advanced chiral stationary phases are reshaping the landscape, promising improved analytical accuracy and regulatory compliance.
Emerging Technologies for Chiral Drug Evaluation
Artificial intelligence (AI) is revolutionizing bioanalytical methods by enabling real-time data processing and predictive modeling. AI-driven bioanalysis aids in the rapid identification of enantiomer-specific pharmacokinetic (PK) patterns, facilitating precise assessments of bioequivalence (Ceramella et al., 2022). Additionally, AI models can optimize experimental designs, reducing resource consumption and trial duration while maintaining analytical rigor.
Machine Learning Applications for Stereospecific Method Development
Machine learning (ML) is increasingly employed to develop and refine stereospecific bioanalytical methods. Algorithms trained on vast datasets can predict the performance of chiral separation techniques, such as high-performance liquid chromatography (HPLC) and liquid chromatography-tandem mass spectrometry (LC-MS/MS), under various conditions. This accelerates method validation and ensures robust enantiomer separation, crucial for regulatory compliance (González-Rojano et al., 2020).
Ethical Considerations in Trial Design
Ethical considerations are paramount in designing chiral bioequivalence trials. Ensuring minimal exposure to ineffective enantiomers and avoiding unnecessary duplication of studies align with ethical guidelines. Moreover, the use of AI and ML in trial design helps optimize participant safety by predicting potential risks and refining dose selection (Torrado et al., 2010).
Opportunities for Regulatory Compliance and Analytical Accuracy
Harmonizing global regulatory standards remains a key opportunity in chiral bioequivalence. Collaborative efforts between agencies like the FDA, EMA, and ICH can streamline requirements, fostering consistency in drug development. Additionally, adopting cutting-edge technologies such as AI-enhanced bioanalysis and advanced CSPs ensures compliance with rigorous regulatory expectations while improving analytical accuracy (Reig-López et al., 2024).
As innovations continue to emerge, the future of chiral bioequivalence is marked by greater precision, efficiency, and collaboration. By integrating AI, ML, and advanced analytical tools, researchers and regulators can enhance the reliability of bioequivalence studies, ultimately improving therapeutic outcomes for patients.
Conclusion
Chiral bioequivalence plays a vital role in modern drug development, ensuring that pharmaceutical formulations meet the highest standards of safety, efficacy, and therapeutic consistency. By focusing on the unique pharmacokinetic (PK) and pharmacodynamic (PD) behaviors of enantiomers, stereospecific bioanalytical methods address critical gaps in the evaluation of racemic mixtures and single-enantiomer drugs. This precision not only enhances the reliability of bioequivalence studies but also safeguards patient outcomes, particularly for drugs where enantiomers exhibit distinct therapeutic and safety profiles.
Key takeaways from advancements in chiral bioequivalence include the importance of analytical precision, the necessity of regulatory alignment, and the profound clinical impact of accurate stereospecific assessments. Techniques such as chiral LC-MS/MS and the development of advanced chiral stationary phases have revolutionized enantiomer-specific analysis, making it more accessible and reliable. Simultaneously, regulatory agencies like the FDA and EMA have recognized the importance of stereospecific evaluations, establishing guidelines to ensure that critical differences in enantiomer behavior are captured effectively.
However, disparities in global regulatory standards present significant challenges, highlighting the urgent need for harmonization. A unified approach, guided by international collaborations such as those facilitated by the ICH, would streamline the drug development process, reduce duplication of effort, and ensure consistent evaluation criteria across regions.
The path forward requires continued investment in stereospecific research and method development. By leveraging innovations like AI-driven bioanalysis and machine learning, researchers can further enhance the efficiency and accuracy of chiral bioequivalence studies. This commitment to advancing the science of stereospecific analysis is essential for fostering innovation in pharmaceuticals and delivering safer, more effective therapeutics to patients worldwide.
In closing, the integration of precise analytical methods, robust regulatory frameworks, and harmonized global standards is not merely a scientific imperative but a moral one. Stakeholders across the pharmaceutical and regulatory spectrum must unite in their efforts to champion these advancements, ensuring that chiral drugs reach their full therapeutic potential for the benefit of all.
Further Reading
Mehvar R, and Jamali F. (1997). Bioequivalence of chiral drugs. Stereospecific versus non-stereospecific methods. Clin Pharmacokinet. 33(2):122-41. doi: 10.2165/00003088-199733020-00004. PMID: 9260035.
Torrado, J. J., Blanco, M., et al. (2010). Rationale and Conditions for the Requirement of Chiral Bioanalytical Methods in Bioequivalence Studies. European Journal of Clinical Pharmacology, 66(6), 599–604.
García-Arieta, A., Gordon, J. (2012). Bioequivalence Requirements in the European Union: Critical Discussion. The AAPS Journal, 14(4), 632–641.
Ceramella, J., Iacopetta, D., Franchini, A., et al. (2022). A Look at the Importance of Chirality in Drug Activity: Some Significative Examples. Applied Sciences, 12, 10909.
García-Arieta, A., Ferrero-Cafiero, J. M., et al. (2016). Impact of Chiral Bioanalytical Methods on the Bioequivalence of Ibuprofen Products Containing Ibuprofen Lysinate and Ibuprofen Base. Chirality, 28(5), 429–433.
González-Rojano, E., et al. (2020). Chiral Bioanalytical Methods in Bioequivalence Studies of Intravenous vs. Oral Formulations of Ibuprofen. Chirality, 32(6), 623–629.
Lees, P., Hunter, R. P., et al. (2012). Pharmacokinetics and Pharmacodynamics of Stereoisomeric Drugs with Particular Reference to Bioequivalence Determination. Journal of Veterinary Pharmacology and Therapeutics, 35(Suppl. 1), 17–29.
Reig-López, J., Cuquerella-Gilabert, M., et al. (2024). Bioequivalence Risk Assessment of Oral Formulations Containing Racemic Ibuprofen Through a Chiral Physiologically Based Pharmacokinetic Model of Ibuprofen Enantiomers. European Journal of Pharmaceutics and Biopharmaceutics, 199, 114293.
Boni JR, et al., (2000). Chiral bioequivalence: effect of absorption rate on racemic etodolac. Clin Pharmacokinet. Dec;39(6):459-69. doi: 10.2165/00003088-200039060-00006. PMID: 11192477.
https://en.wikipedia.org/wiki/Chiral_analysis