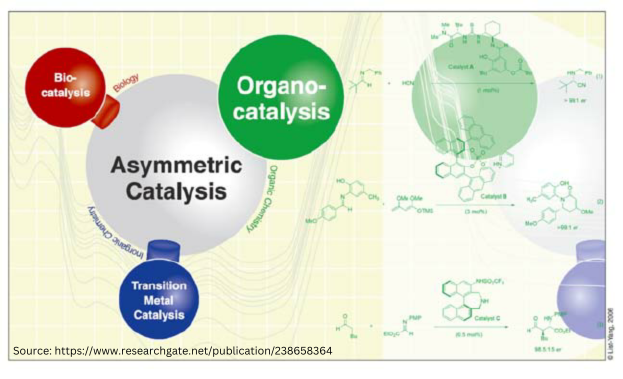
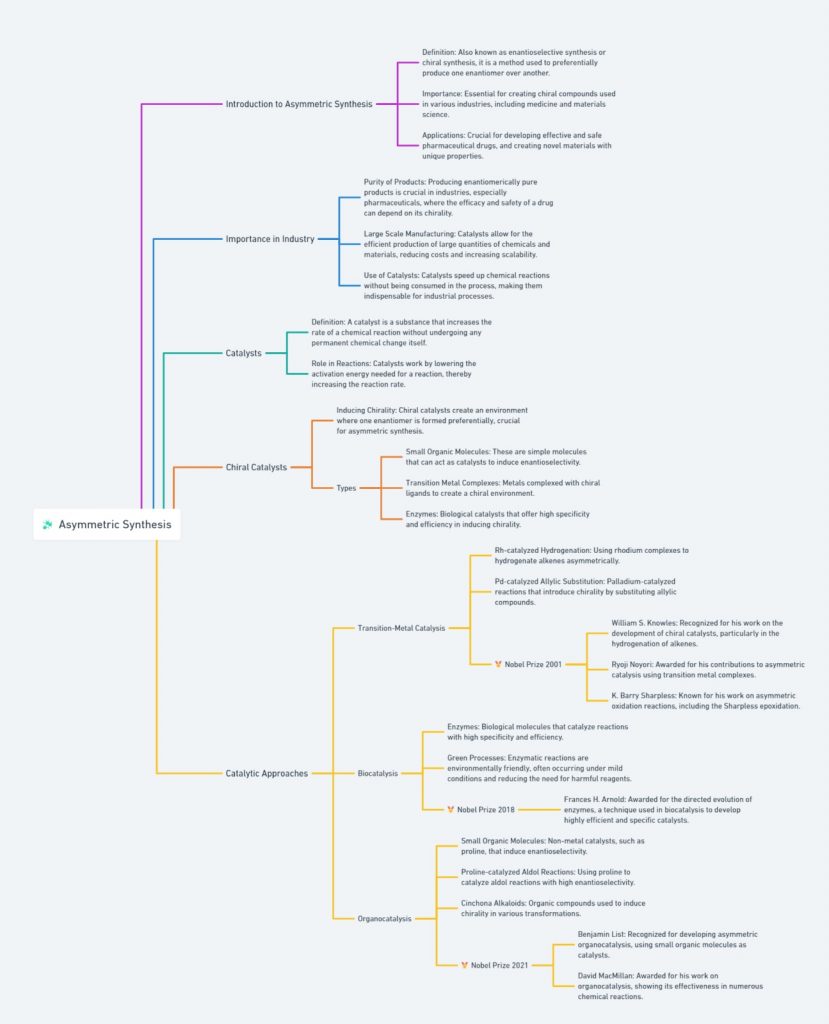
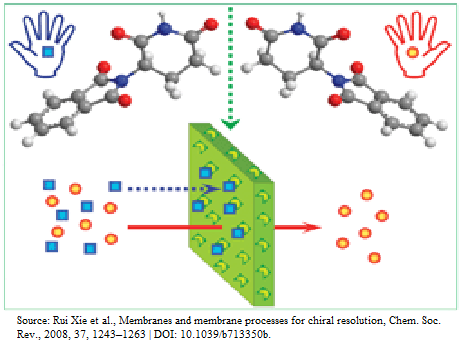
Chiral Drugs: A twisted tale in pharmaceuticals
Synopsis Chiral drugs, those fascinating chemical compounds existing as mirror-image pairs or enantiomers, exhibit distinct biological properties. This post provides a comprehensive overview of chiral drugs, presenting key concepts through a visually engaging mind maps. It covers chirality’s historical roots and stereochemical nomenclature, delves into the specialized discipline of chiral pharmacology, and presents drug toxicity case studies. Additionally, it explores unichiral drugs, the importance of chiral purity, and the tools used for measuring it. Designed …