“Beyond today – emerging trends shaping the future of stereochemistry in pharma”
Introduction
The landscape of stereochemistry in pharmaceutical sciences continues to evolve with scientific and technological advances. This final part looks at emerging trends and future challenges. These include: – New developments in asymmetric catalysis, such as organocatalysts (MacMillan/List catalysts) that have expanded the toolkit for chiral synthesis, and the 2021 Nobel recognition of this field – implying more green and metal-free asymmetric processes in drug manufacturing. – AI and machine learning in stereochemistry: from predicting enantioselective reaction outcomes to designing chiral drug candidates with desired stereochemistry, and using computational tools to propose chiral catalysts (some research uses algorithms to design ligand structure for a desired stereochemical outcome). – Automation and high-throughput experimentation for chiral catalyst screening – enabling faster development of asymmetric reactions (some companies have catalyst libraries that can be rapidly screened by robots to find best conditions, which accelerates obtaining an asymmetric route). – Green chemistry approach: an emphasis on catalytic and enzymatic methods over resolution (to minimize waste), and using biocatalysis in place of heavy metals (as noted by trends at companies like BASF. We highlight examples like enzyme engineering allowing replacements of traditional stoichiometric reagents (e.g., transaminases replacing resolving of racemic amines). – Regulatory developments: The possibility of changes in guidance reflecting growing capabilities – maybe future guidelines will push even harder for single enantiomers (though they already do indirectly) or incorporate new analytical standards (e.g., maybe requiring chiral MS for certain cases). – Stereochemistry and personalized medicine: Not directly obvious, but one can consider that different patient populations might metabolize enantiomers differently (pharmacogenetics we touched on – e.g., CYP2C9 alleles and warfarin). Future therapy might consider not just the drug but also which enantiomer is optimal for an individual (though in practice, you’d just choose one globally if it’s better). – 3D printing of drugs – if we think far future, could one print a drug in a specific chiral form? Likely one would print using already synthesized enantiopure materials, so nothing new stereochemically, but interesting concept: printing might allow mixing in of chiral excipients like cyclodextrins differently?
- Atropisomerism and conformational chirality: An emerging focus in medicinal chemistry is designing molecules with restricted rotation (atropisomers) and considering them as separate entities. Some drug candidates might have axial chirality (e.g., kinase inhibitors with biaryl axes that are atropisomers – sometimes one axial isomer is more active). Historically these were sometimes made as racemates because separating was tough, but now folks are developing atroposelective syntheses or isolating the stable atropisomers. The FDA has started treating stable atropisomers like ordinary enantiomers regarding characterization. Future might see more explicitly chiral axes in drugs (the “3D” push might yield more atropisomeric scaffolds).
- Mirror-image biologics (just speculative): Perhaps someone in future will develop a mirror-image protein therapeutic that avoids immune detection. Already, Spiegelmers (mirror aptamers) have entered clinical trials (e.g., NOX-A12 by Noxxon Pharma), showing the concept. Maybe a mirror-image antibody one day? That’s extremely challenging (need chemical synthesis of a large D-protein – not feasible with current tech, but maybe smaller D-peptides as antibody mimetics?).
Advances in Catalysis and Synthesis
We mention 2021 Nobel prize to List and MacMillan which underscores how organocatalysis is now mainstream. Many asymmetric organocatalytic reactions have been discovered (e.g., chiral iminium catalysis for Diels-Alder and Michael, chiral phase-transfer catalysis for alkylations, etc.). Industry adoption of organocatalysis is growing because these catalysts are often low toxicity and can be cheaper (no precious metals). Also new trends: – Photoredox catalysis combined with chirality – there have been very recent breakthroughs in merging visible light catalysis with chiral catalysts to do radical asymmetric reactions (e.g., MacMillan’s 2017 Science paper on asym photoredox). This opens radical chemistry to asymmetric control, which was hard before. Could allow making chiral centers adjacent to complex structures more easily. – C-H activation – making sp3-sp3 bonds in presence of chirality. There are new chiral catalysts to do enantioselective C-H functionalizations (like Yu and others have done directed C-H insertion with chiral transient directing groups). – Flow chemistry and chirality: continuous flow reactors can sometimes improve asymmetric reaction outcomes by better control, and allow scaling of sensitive catalytic processes.
AI in Stereochemistry
Machine learning is being applied to predict enantioselectivity of catalysts (there are studies where ML models predict ee given substrate and catalyst parameters). Also generative models could propose chiral molecules with specific desired shapes or docking poses. In drug design, perhaps AI could enumerate stereoisomers of a scaffold and test them in silico quickly to suggest which to synthesize. Actually, some docking programs automatically consider all stereoisomers of a given ligand if not specified, and can rank them. Another area: retrosynthesis planning AI – these tools now incorporate options for asymmetric steps when needed. E.g., if making a target requires a chiral center, the AI might suggest a known asymmetric hydrogenation step from literature.
Green Chemistry & Sustainability
There’s regulatory and environmental pressure to reduce use of heavy metals and large amounts of solvents. Asymmetric catalysis helps since it’s catalytic (less waste) and organocatalysts or enzymes avoid heavy metals in waste streams. The pharma industry is adopting biocatalysis for chiral steps (like Merck with sitagliptin transaminase case, or using lipases for ester resolutions). We foresee more synthetic routes having one or multiple enzyme steps – essentially harnessing nature’s stereoselectivity in manufacturing. Also, dynamic kinetic resolutions (combining chemical racemization with enzymatic resolution) to get full yield with minimal waste is an elegant synergy that might be more used.
Regulatory Challenges
As compounds get more complex (some new drugs have multiple stereocenters and even conformational isomers), regulators may challenge companies to fully characterize those. E.g., a drug with two chiral centers has 3 diastereomers plus enantiomer of one if racemic at one center – if one is drug and another diastereomer is present as impurity, need to quantify and control it. Tools like chiral HPLC can separate diastereomers easily usually, but if something has atropisomers that interconvert slowly, how to deal? The concept of “stereo chemical purity” might extend beyond just enantiomeric purity to also “conformational purity.” Possibly in future, guidelines will mention atropisomerism and require control if at room temp the half-life of interconversion is long relative to shelf-life or dosing time. Already ICH Q7A states one should control stereochemical quality of drug.
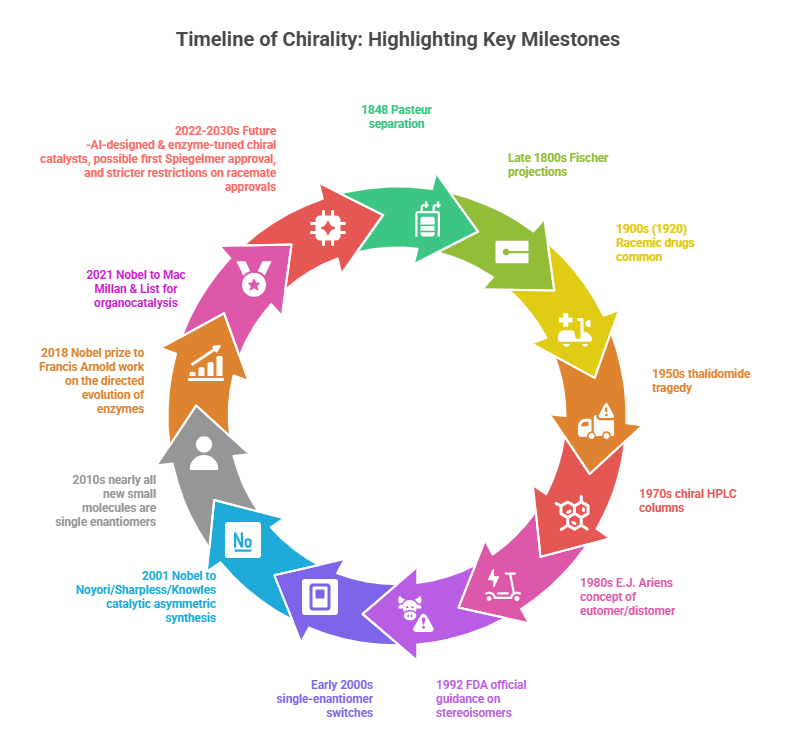
Timeline
A timeline highlighting key milestones in the development of chiral chemistry.
Future Challenges
One challenge remains synthesizing molecules with many stereocenters efficiently. Even with all tools, it’s non-trivial if no symmetry or repeating units. Perhaps semi-synthesis or using nature’s help (fermentation or chemoenzymatic) will be employed more. Another challenge: addressing chirality of chiral excipients (like some polymer or cyclodextrin excipients are chiral; normally both enantiomer will have same physical function but if an excipient interacts biologically, its chirality might matter). Additionally, as computing gets better, we might see more in silico modeling of chiral recognition (like simulating how enantioselective binding happens in a catalyst or receptor, to design better catalysts or drug enantiomers rationally).
Summary (Part 10)
– Asymmetric synthesis methods continue to advance (organocatalysis, photo-redox, biocatalysis), making it easier and greener to obtain pure stereoisomers. Future drug syntheses will likely incorporate these to reduce reliance on classical resolution and cut waste.
– AI/ML is emerging as a tool to tackle stereochemical problems: e.g., predicting which chiral ligand will give high ee in a reaction, or designing chiral molecules with desired shape for a target (computational chemists now use algorithms to generate conformationally complex structures, addressing what was historically a bias towards flat, aromatic scaffolds).
– The push for 3-dimensionality in drug discovery means more compounds with chiral centers, chiral axes, or helices will be considered – requiring medicinal chemists to manage stereochemistry from the earliest stages. We might see more instances where drugs have interesting chirality (like a drug that is a chiral helquat (helical cation) or a planar chiral metallocene derivative, etc., exploring novel chemical space).
– Regulatory framework will keep pace: ensuring any new type of chirality (atropisomers, etc.) is properly evaluated. The definition of “stereochemical purity” in regulatory filings may broaden beyond just enantiomeric purity to “isomeric purity” including any stereoisomers. – In drug delivery, perhaps chiral nanoparticles or chirally surface-modified delivery systems could become relevant (e.g., a lipid nanoparticle where one enantiomer of a lipid fosters better interaction with cell membranes).
– Chiral sensing and diagnostics – using chiral drug molecules or excipients as sensors for biomolecules (some devices can use chiral coatings to detect enantioselective interactions, though that’s more analytical instrumentation).
Suggested Reading
https://www.nobelprize.org/prizes/chemistry/2021/popular-information
List, B. (2021). “My Adventures in Asymmetric Catalysis.” Angew. Chem. Int. Ed. (Nobel lecture) – outlines how organocatalysis came and future perspectives.
Patricia Van Arnum, Single-Enantiomer Drugs Drive Advances in Asymmetric Synthesis, Pharmaceutical Technology, 2006,
Volume 30, Issue 4. https://www.pharmtech.com/view/single-enantiomer-drugs-drive-advances-asymmetric-synthesis
OpenAI or DeepLearning in chemistry papers on predicting enantioselectivity, e.g., Granda et al. (2018) Nature, 559, 377 (on using AI to predict outcomes of organic reactions including stereochem).
Shevlin, M. (2018). “Practical High-Throughput Experimentation for Chemists.” ACS Med. Chem. Lett., 8, 601-607. (Discusses how automation is used in pharma including screening chiral catalysts quickly).
Trends article: “Atropisomerism in Medicinal Chemistry” (2019, J. Med. Chem. or similar) – summarizing how pharmaceutical industry is dealing with axial chirality and recent drugs with atropisomerism.
Regulatory watch: EMEA or FDA commentary on single enantiomer drugs vs racemates around late 2000s – though they mostly refer to existing guidance. Possibly a recent FDA blog on use of biosynthesis and green methods in pharmaceuticals reflecting industry shift.