“Nature’s stereochemists – chirality in biologics, peptides, and natural products”
Introduction
Stereochemistry is inherent in biological macromolecules and natural products. This part explores chirality beyond small synthetic drugs – specifically, in biologics (peptides, proteins, nucleic acids) and in natural product-derived drugs. We examine how nature’s biosynthetic machinery imparts stereochemistry with high fidelity (e.g., enzymes produce single enantiomers of amino acids, sugars, complex polyketides). We discuss examples of drugs that are derived from natural chirality (like antibiotics, alkaloids, terpenes) and the challenges in synthesizing or modifying such molecules. Additionally, we consider cases where non-natural stereochemistry is introduced in biologics for improved properties (e.g., D-amino acids in peptide drugs to resist proteolysis). The concept of life’s “homochirality” is addressed – all natural proteins are L-amino acid-based, all DNA/RNA have D-sugars – and implications of that for drug design (e.g., why L-peptides vs D-peptides behave differently in the body).
Chirality in Peptides and Proteins
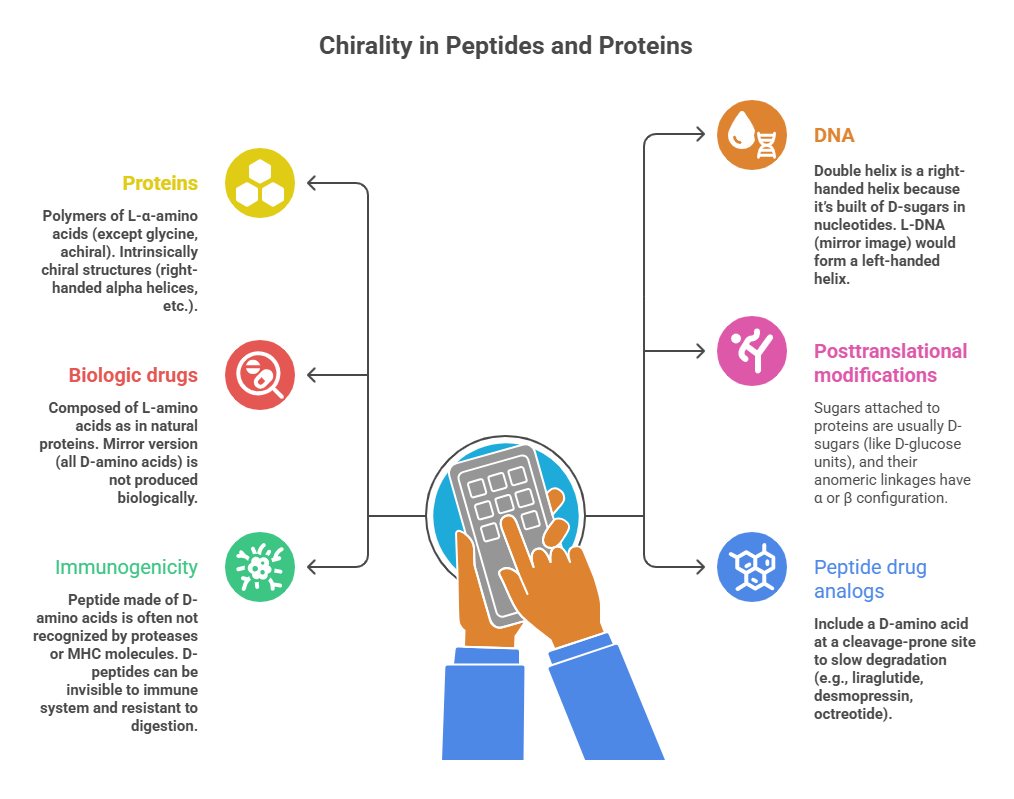
Proteins are polymers of L-α-amino acids (except glycine, achiral) – thus they are intrinsically chiral structures (right-handed alpha helices, etc.). The entire architecture of a protein (secondary, tertiary structure) is dependent on the stereochemistry of its building blocks. For example, DNA’s double helix is a right-handed helix because it’s built of D-sugars in nucleotides. If one made an L-DNA (mirror image), it would form a left-handed helix (and indeed, such “mirror DNA” has been studied in labs). In terms of therapeutics:
– Biologic drugs like monoclonal antibodies, insulin, growth factors – these are composed of L-amino acids as in natural proteins. One doesn’t have “enantiomeric forms” of these because the mirror version (all D-amino acids) is not produced biologically. (Though conceptually, a mirror image protein could be synthesized chemically – and some research into mirror-image peptides for drugs exists, but not mainstream). – The stereochemistry in biologics is controlled by biosynthesis – ribosomes incorporate only L-amino acids (with rare exceptions in bacteria that incorporate D-amino acids in some small peptides via special mechanisms). So, while small synthetic drugs can be racemic, biologic drugs are essentially produced as a single stereoisomer (e.g., only one chiral form of epoetin alfa exists as it’s a specific amino acid sequence of defined chirality).
However, posttranslational modifications (glycosylation, etc.) also have stereochemical aspects – sugars attached to proteins are usually D-sugars (like D-glucose units), and their anomeric linkages have α or β configuration that matters for recognition (though glycan chirality often remains consistent – enzymes that add sugars do so in specific orientation).
One interesting point is immunogenicity: our immune system is tuned to natural L-proteins. A peptide made of D-amino acids is often not recognized by proteases or MHC molecules the same way, meaning D-peptides can be invisible to immune system and resistant to digestion. This is why some peptide drug analogs include a D-amino acid at a cleavage-prone site to slow degradation (e.g., liraglutide, an analog of GLP-1, has all L-amino acids but is fatty acylated and not sure if any D-AA is in there; but another example: desmopressin (DDAVP) is an analog of vasopressin where one L-amino acid (at position 8) is replaced with D-arginine, hence “D” in name, which greatly extends its half-life). Another example: octreotide (a synthetic octapeptide drug) contains D-tryptophan and D-phenylalanine, which help it maintain conformation and resist enzymatic breakdown, giving it a much longer duration than the natural somatostatin (which is all L and very short-lived).
So, paradoxically, while our biologic drugs (like therapeutic proteins) stick to natural chirality, some peptide drugs (which are sort of between small molecules and biologics) intentionally incorporate unnatural stereochemistry to improve performance.
Chirality in Nucleic Acids
DNA and RNA have D-2-deoxyribose and D-ribose sugars respectively (which in Fischer terms correspond to a certain configuration historically labeled D due to relation to glyceraldehyde; note D in sugar nomenclature is a stereochemical descriptor not directly meaning dextrorotatory for all sugars). These sugars have several stereocenters – DNA’s monomers are each chiral (at least 4 chiral centers in each nucleotide sugar). Nature uses one enantiomer of each building block exclusively (except rare organisms using L-RNA? So far life uses D-sugars in genetic material). From a drug perspective, aptamers (nucleic acid therapeutics) are typically composed of natural D-RNA or D-DNA, but there is research in mirror aptamers (“Spiegelmers”) which are made of L-nucleotides. L-oligonucleotides would not be recognized by nucleases (thus very stable in body), and won’t hybridize with natural nucleic acids (so low off-target risk related to genome). The tricky part is to find an L-aptamer that binds a target, one can perform SELEX on the mirror image of the target using D-oligonucleotides (as the mirror of a mirror yields original orientation interactions). This is advanced medicinal chemistry exploited by some companies. It’s a fascinating intersection of chirality in large molecules.
Chirality in Natural Products
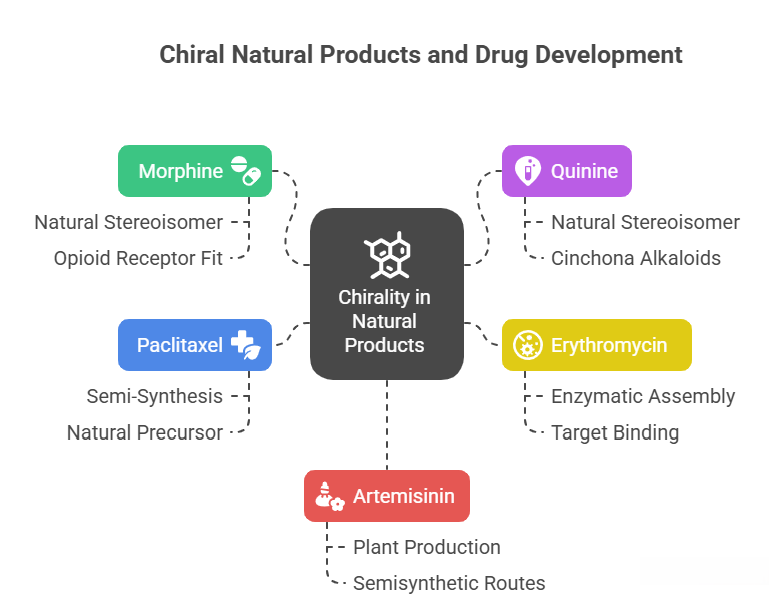
Most complex natural products (alkaloids, terpenes, polyketides, etc.) are chiral and are found in nature usually as single enantiomers (because enzymes produce one configuration). For example: – Morphine: It has 5 chiral centers, and only one stereoisomer (the natural one) is produced by the opium poppy. That one (with the specific 5R,6S,9R,13S,14R configuration given) is the active narcotic analgesic. The mirror image of morphine, if one could synthesize it, likely would not fit the opioid receptor as well (and indeed it’s not found in nature). Interestingly, slight modifications like codeine, etc., maintain those stereocenters. – Quinine: 4 chiral centers, only one stereoisomer (3R,4S,8S,9R as given) is natural and medicinal (antimalarial). Its enantiomeric and other diastereomeric forms are different Cinchona alkaloids (like quinidine is the epimer at C8 relative to quinine). – Many antibiotics (like erythromycin, vancomycin) are loaded with stereocenters set by enzymatic assembly lines. These are all crucial for their binding to targets (like vancomycin binds D-Ala-D-Ala in cell walls; its own shape is highly chiral and complementary to that peptide terminus).
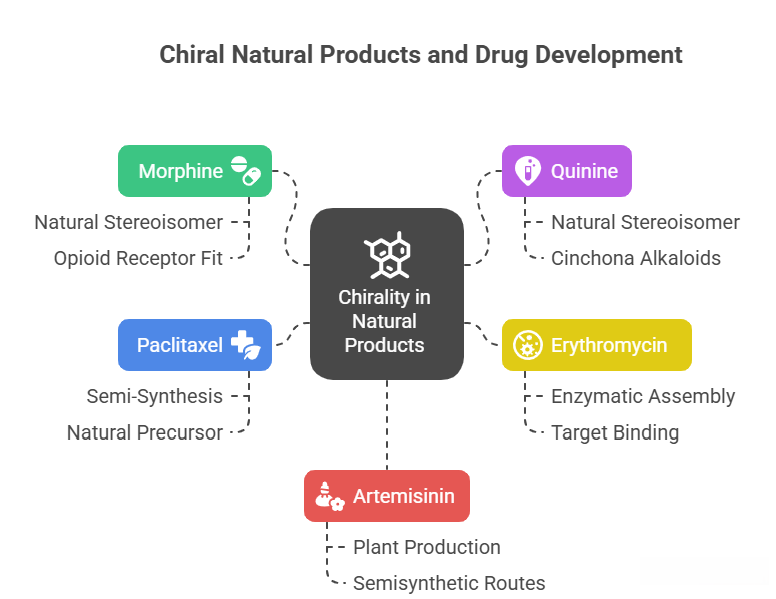
For drug development, when a natural product is the lead: – If it’s plentiful from nature, you isolate it as that single stereoisomer. – If you need to synthesize it (due to supply issues or to create analogs), you face a daunting stereochemical synthetic challenge. Often chemists will utilize chiral pool or enzyme steps for part of it. For example, Paclitaxel (Taxol) has 11 stereocenters; total synthesis is extremely complex. Instead, it’s semi-synthesized from a natural precursor (10-deacetylbaccatin) from yew trees – thereby leveraging nature’s stereochemical assembly and just adding side chain via a few steps (which themselves require stereochemical control, but far fewer). – Another: Artemisinin (antimalarial) has multiple stereocenters. It’s produced by the plant Artemisia annua with those stereocenters fixed (it’s a sesquiterpene lactone). Synthetic routes like “semisynthesis” start from artemisinic acid (biosynthetic precursor from engineered yeast) and photochemically transform it to artemisinin, preserving the chiral core introduced by yeast. – Erythromycin and its derivatives (azithromycin, clarithromycin) – early on supplied by fermentation (Streptomyces). Later modifications (like making semisynthetic analogs) maintain the core’s stereochemistry; no attempt to make unnatural enantiomer – likely it wouldn’t work as antibiotic because it must fit the ribosome.
Enzymatic Stereocontrol (Biosynthesis):
Why are natural product molecules often so stereochemically complex and single-enantiomer? Because enzymes are chiral catalysts that form specific stereochemical outcomes – either by chiral active sites or by chiral auxiliaries (like cofactors or protein scaffolds). – E.g., polyketide synthases have acyl carrier proteins with chiral phosphopantetheine arms and enzyme domains that reduce and cyclize intermediates in defined orientations, leading to specific hydroxyl stereochemistry or double bond geometry. Thus, erythromycin has all centers set certain way by the PKS and tailoring enzymes. – Terpene cyclases take an achiral substrate (like farnesyl diphosphate) and convert it into complex chiral skeletons like that of artemisinin precursor, in a single enzyme step with induction of multiple stereocenters in one go, all controlled by the enzyme’s shape.
Case Studies from Natural Product Drugs: – Morphine (from Papaver somniferum): Only (-)-morphine is natural; interestingly, the + enantiomer has been synthesized in research and found virtually inactive at opioid receptors or with very different properties (it can actually act as NMDA antagonist rather than opioid agonist). – Thalidomide itself is a synthetic drug but as a side note: it originally was a mixture but we know one enantiomer’s different effect – even though it’s synthetic, it’s a cautionary tale that relates to how a chiral environment (the developing embryo’s chiral biochemistry) interacted differently with each enantiomer. – Paclitaxel: glean one example from text: It has numerous chiral centers, any deviation and it loses activity. The unnatural enantiomer of paclitaxel has been a theoretical curiosity but no one tries to make that – it’s expected to fail binding to tubulin or not fit properly (plus enormous synthetic effort would be needed). – Penicillin G: It has 3 stereocenters and is produced by fermentation as a single stereoisomer (6-APA core has those defined). The entire beta-lactam mechanism partly relies on those stereochemistry to mimic D-Ala-D-Ala.
Unichirality of Life
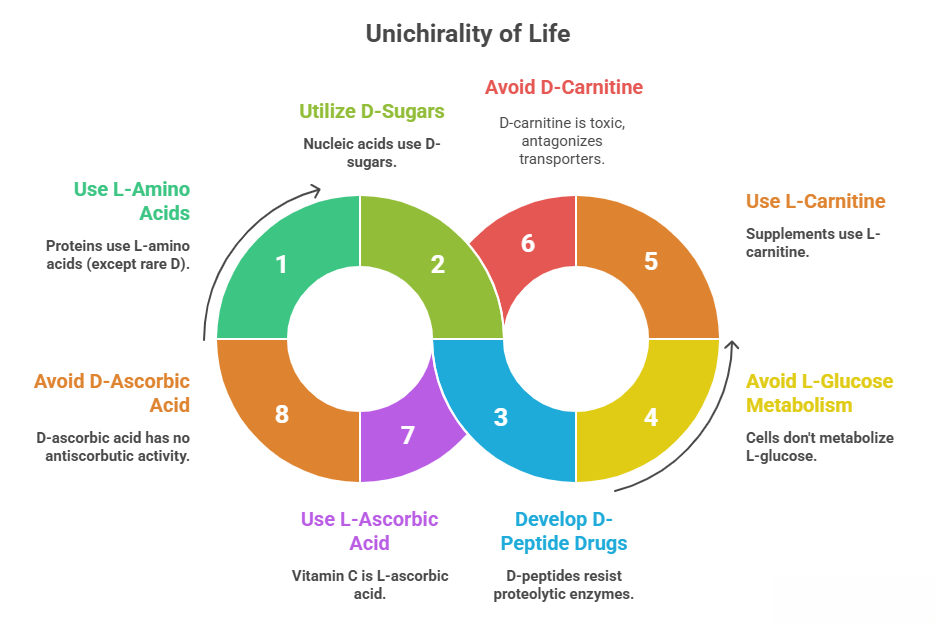
All amino acids in proteins are L (except rare D in bacterial cell walls or nonribosomal peptides), all sugars in nucleic acids are D. This uniform chirality is thought to be fundamental to how biomolecular interactions are specific. If a drug is a peptide composed of D-amino acids, it typically will not be recognized or processed by proteolytic enzymes (because those enzymes evolved for L-substrates). This is exploited for stability (e.g., tertible D-peptide HIV entry inhibitors being researched; they don’t get degraded and have long half-lives). But it also means if one tried to, say, feed cells with D-glucose only, they wouldn’t metabolize it (they need the usual D-glucose because enzymes are specific – actually wait, they metabolize D- but not L-glucose, as L is unnatural to them. Correction: cells use D-glucose, the naturally occurring sugar in diet, and L-glucose is what’s not metabolized). Yes, life is fine-tuned to one enantiomer of key metabolites: – L-amino acids: used in protein synthesis, D-amino acids not used except specialized cases. – D-sugars: in metabolism (most sugars in glycolysis are D, like D-glucose, D-fructose; interestingly, “D” in sugar names historically indicates configuration relative to D-glyceraldehyde, which for glucose means the OH on penultimate carbon is on right in Fischer – those are the naturally occurring. L-sugars exist but not typically in major metabolism). Thus, any drug interacting with these systems needs to consider that: If you want a molecule to avoid metabolic pathways, making it the unnatural enantiomer can do that. Eg: L-carnitine is the natural enant used in body; D-carnitine is not utilized and can even cause harm by antagonizing transporters. This is why carnitine supplements must be L-carnitine; the D isomer is considered toxic (it can inhibit activity of L-carnitine in body, leading to deficiency). Another: ascorbic acid (Vitamin C) is L-ascorbic acid (from glucose in plants, ironically L by nomenclature but laevorotatory is known as vitamin C), D-ascorbic acid has no antiscorbutic activity.
Synthesis of Chiral Natural Molecules
Due to difficulty, often semisynthesis or fermentation is used. In some cases, total synthesis of a natural product drug is achieved using chiral catalysis or auxiliaries. Eg: quinine was famously synthesized by R.B. Woodward in 1944 (with some stereoselective steps, albeit in poor yield). Nowadays, many natural product derivatives (like eribulin, an anticancer derived from halichondrin B) are made by partial synthesis or heavily rely on chiral resolution.
Summary (Part 8)
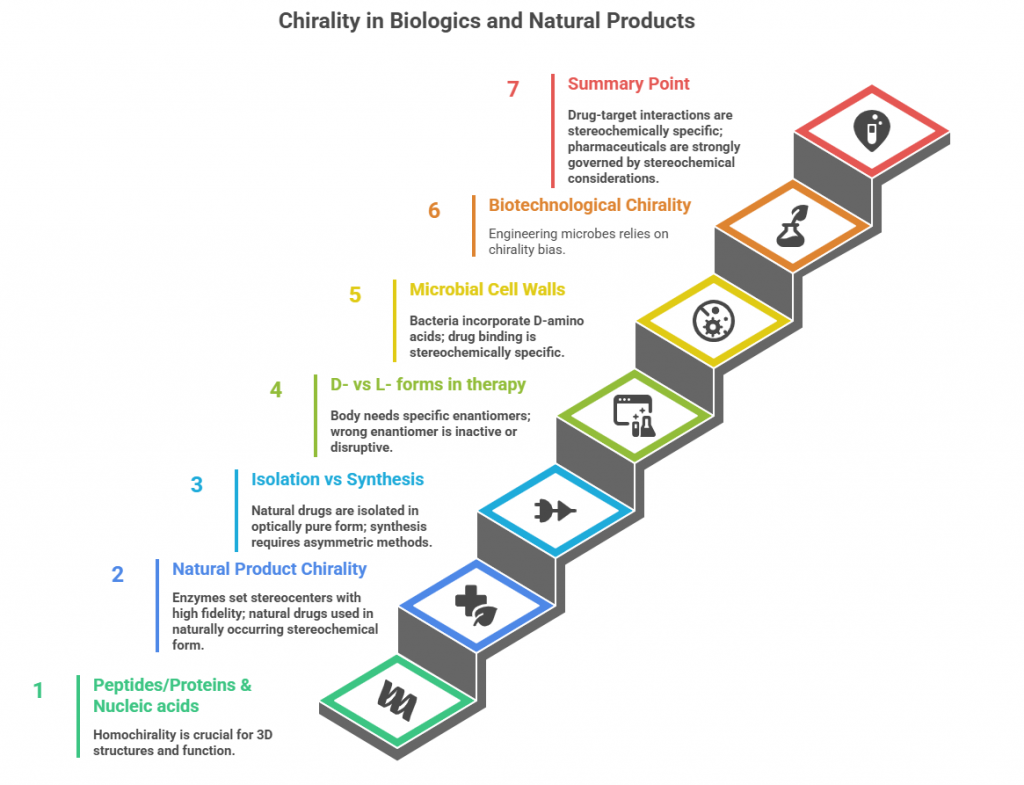
– Peptides/Proteins: Natural proteins are homochiral polymers of L-amino acids, giving rise to uniformly chiral 3D structures (e.g., right-handed helices) that are crucial for function. Biologic drugs thus have defined stereochemistry inherently. Unnatural D-amino acids in proteins aren’t incorporated by ribosomes, but adding D-amino acids in synthetic peptides can greatly alter properties (usually to increase stability to proteolysis or reduce immunogenicity). – Nucleic acids: Also homochiral (D-sugars in backbone). Mirror-image nucleic acids (L-DNA, L-RNA) are being explored for therapeutics due to their biostability and unique properties (Spiegelmers). – Natural product chirality: Life typically produces one enantiomer of a chiral compound. Enzymes in biosynthetic pathways set multiple stereocenters in complex natural products with high fidelity (often >99% e.e. essentially, since one enantiomer is made exclusively). Natural products used as drugs (paclitaxel, vinca alkaloids, macrolide antibiotics, etc.) are usually used in that naturally occurring stereochemical form because the biological activity is tied to that specific 3D arrangement. The other enantiomer or diastereomers if made would likely be far less active or interact differently (e.g., the unnatural enantiomer of an antibiotic often won’t bind its target). – Isolation vs Synthesis: Many chiral natural drugs are isolated from organisms, which deliver them in optically pure form. If chemical synthesis is undertaken, stereochemistry must be introduced by asymmetric methods or using chiral starting materials from nature (chiral pool). Example: semi-synthesis of paclitaxel uses naturally extracted baccatin III (with its stereocenters preset). – Case examples: Morphine only one stereoisomer has analgesic activity; Quinine vs quinidine (diastereomer) have different medical uses (quinidine as antiarrhythmic, quinine as antimalarial) showing how different stereochemistry even on same connectivity leads to different bioactivities. – D- vs L- forms in therapy: For vitamins, amino acids, etc., the body often needs a specific enantiomer (L-amino acids for protein, D-sugars for metabolism, etc.). Wrong enantiomer is inactive or can even disrupt normal processes. E.g., D-amino acids in diet are mostly not used (and in some cases can have osmotic or other effects). L-ascorbic acid is vitamin C, D-ascorbic is not biologically equivalent. – Microbial cell walls and stereochemistry: Bacteria incorporate D-amino acids (like D-Ala) in peptidoglycan; interestingly, vancomycin binds to D-Ala-D-Ala termini – an example where a drug (vancomycin, a natural glycopeptide) is a chiral molecule specifically complementing the stereochemistry of a bacterial target (which uses D-Ala). If the bacteria switch one D-Ala to D-lactate (slightly different stereochemistry or just replacement), vancomycin binding is lost (mechanism of resistance). That underscores how precise these lock-and-key interactions are stereochemically. – Biotechnological chirality: When engineering microbes to produce drug precursors, one often relies on their chirality bias. Eg: engineered E. coli to produce L-valine (only L form is produced by its enzymes). Synthetic biology is also creating non-natural compounds by tweaking enzyme specificity, but typically the products remain chiral in ways similar to the original path (though occasionally one can invert a stereocenter by mutating an enzyme). Summary point: Life’s systems are chiral, so all interactions (drug-target, enzyme-substrate) are stereochemically specific. This is why chirality is so important in pharmacology – a drug must fit into chiral binding sites of proteins. Natural evolution has resolved to single enantiomers likely because a mix would be less efficient (ensuring uniform chirality improves consistency in building blocks). As a result, pharmaceuticals that interface or originate from biological systems are strongly governed by stereochemical considerations.
Suggested Reading
1. “Chirality and Life: A Short Review” – G. Pályi et al., Chirality, covers the concept of biomolecular homochirality and its significance in biochemistry.
2. V. Schirrmacher et al. (2009). “Mirror-image biomolecules: D-amino acid peptides and L-DNA in medical research.” Drug Discovery Today, 14(7-8), 314-322. (Discusses mirror-image peptides (Spiegelmers) for therapeutics.)
3. “Natural Product Biosynthesis” by C. Walsh & Y. Tang, specifically chapters on how enzymes set stereochemistry in polyketides and nonribosomal peptides (for insight into e.g., erythromycin or vancomycin’s stereochem).
4. M. Egorov (2019). “D-Amino Acid Containing Peptides: Bioactivity and Therapeutic Potential.” AAPS J., 21:83. (On roles of D-AAs in peptides and drug examples like oxytocin analogs, etc.)
5. K. C. Nicolaou’s accounts on total synthesis of complex natural products, e.g. Angew. Chem. 2014, “Adventures in Total Synthesis: The Enduring Appeal of Complex Natural Products,” which often include discussion of tackling stereochem via creative methods.
https://en.wikipedia.org/wiki/Sharpless_epoxidation
Vater A, Klussmann S. Turning mirror-image oligonucleotides into drugs: the evolution of Spiegelmer(®) therapeutics. Drug Discov Today. 2015 Jan;20(1):147-55. doi: 10.1016/j.drudis.2014.09.004.