“The 3D language of molecules – why stereochemistry is the hidden key in drug science.”
Introduction
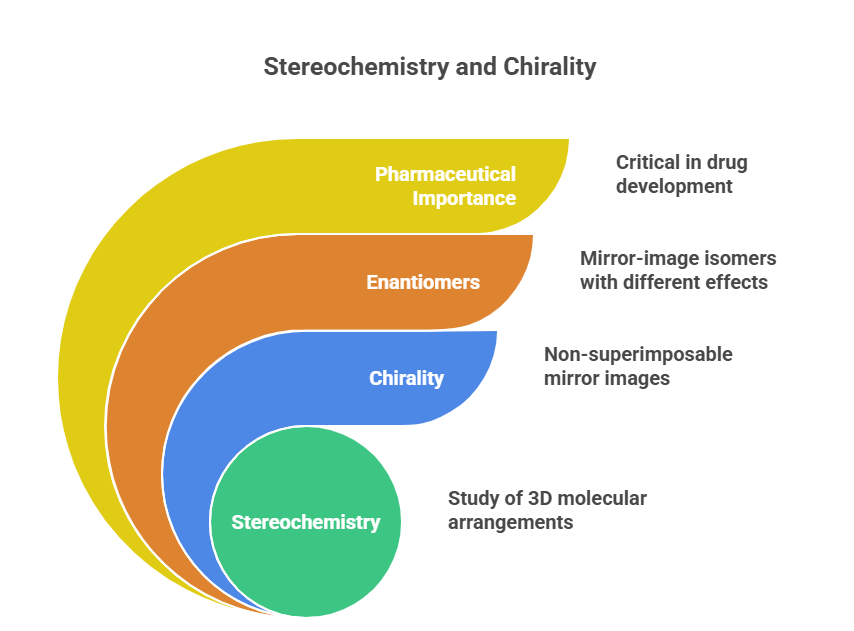
Stereochemistry is the branch of chemistry concerned with the three-dimensional arrangement of atoms in molecules and how this spatial arrangement influences chemical behavior. The term “chirality” (from the Greek cheir meaning hand) describes the property of an object or molecule that is non-superimposable on its mirror image – much like one’s left and right hands. A molecule that exhibits chirality is said to be chiral, whereas one that can be superimposed on its mirror image (often due to an internal plane of symmetry) is achiral. Chirality is a pervasive phenomenon in chemistry and biology: as Lord Kelvin first noted in 1894, it spans scales from subatomic particles to biomolecules and even galaxies. In the context of pharmaceuticals, stereochemistry has enormous importance because enantiomers (mirror-image isomers) of a drug can have drastically different effects in biological systems. A classic example is the tragedy of thalidomide: one enantiomer of the drug had the desired sedative effect, while its mirror-image caused severe birth defects. This underscores why understanding and controlling stereochemistry is critical in drug development. (Consult following blog for a detailed read @ https://chiralpedia.com/blog/the-building-blocks-of-stereochemistry/)
Chiral vs. Achiral Molecules
A molecule is chiral if it cannot be superimposed on its mirror image by any combination of rotations or translations. Typically, chirality in organic molecules arises from a chirality center: a tetrahedral atom (often carbon) bonded to four different substituents. For example, lactic acid (2-hydroxypropanoic acid) has a chiral center (the central carbon bonded to –OH, –CH3, –COOH, and H) and exists as two enantiomers, whereas propionic acid (which lacks such a center) is achiral. An achiral molecule has at least one element of symmetry (such as a mirror plane) that makes it superposable on its mirror image.
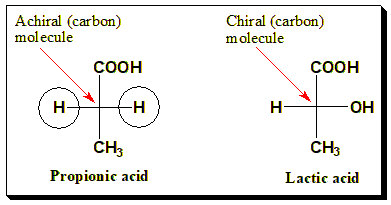
A simple achiral example is glycine, the only amino acid without a chiral center (its side chain is a hydrogen). In contrast, alanine (with a methyl side chain) is chiral and exists as D- and L-alanine enantiomers.
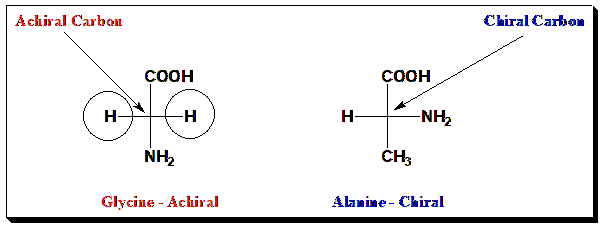
The above Figure depicts the structure of Glycine and Alanine in Fischer projection for comparison. A chiral molecule versus an achiral molecule – for instance, Alanine (chiral) compared to Glycine (achiral). By manipulating rotatable models of each, one can see that alanine’s mirror image cannot be aligned with itself (confirming chirality), while glycine’s can. This visual aid helps learners grasp the “handedness” concept of chirality in a tangible way.
Historical Perspective
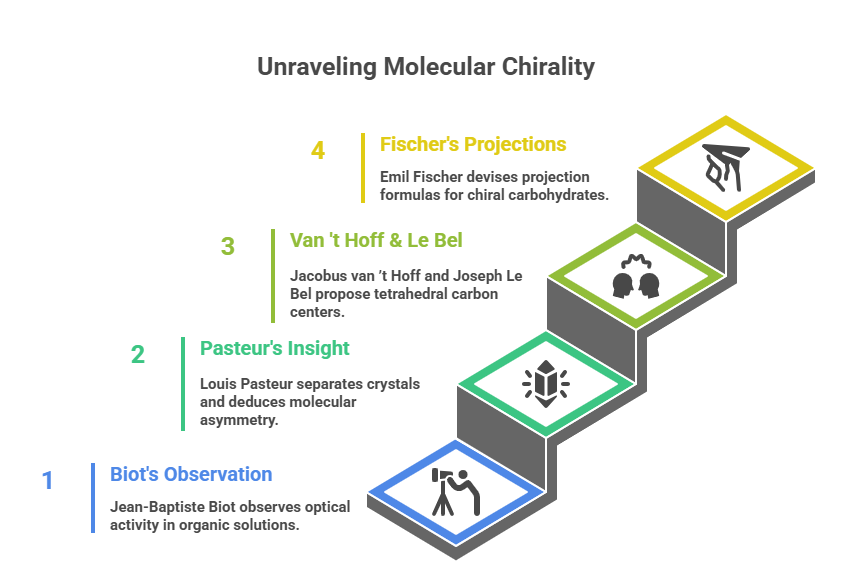
The concept of molecular chirality has its roots in the 19th century. Jean-Baptiste Biot observed in 1815 that certain organic solutions (e.g. tartaric acid) rotate plane-polarized light, a phenomenon termed optical activity. However, it was not until 1848 that Louis Pasteur provided a molecular explanation. Pasteur painstakingly separated two types of crystals of sodium ammonium tartrate – noting they were mirror-image forms – with tweezers, and found each type rotated plane-polarized light in equal magnitude but opposite directions. He deduced that the molecules themselves must be asymmetric (having “left-handed” and “right-handed” forms). Pasteur’s insight – that molecules can have mirror-image forms with identical composition but different 3D arrangement – marks the birth of stereochemistry. It was later formalized by Jacobus van ’t Hoff and Joseph Le Bel (in 1874) with the proposal of tetrahedral carbon centers, and by Emil Fischer, who devised projection formulas (Fischer projections) to depict chiral carbohydrates on paper. (For a detailed discussion on the history of chirality refer blog @ https://chiralpedia.com/blog/the-origins-of-chirality-from-light-to-life/)
Structural Representations
Chemists use several conventions to depict 3D molecular structures in two dimensions while conveying stereochemical information:
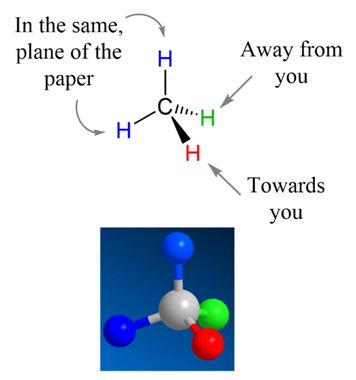
Wedge-Dash Notation:
This is a common drawing style in which a solid wedge indicates a bond coming out of the plane toward the viewer, and a dashed/hashed wedge indicates a bond going behind the plane away from the viewer. For example, in a tetrahedral chiral center, one can draw two bonds in the plane (as ordinary lines), one as a wedge (front), and one as a dash (rear). Wedge-dash depictions allow us to see the 3D orientation at a glance. (A diagram showing a chiral carbon with substituents oriented using wedge and dash bonds, illustrating how rotating the molecule in space corresponds to the 2D depiction.)
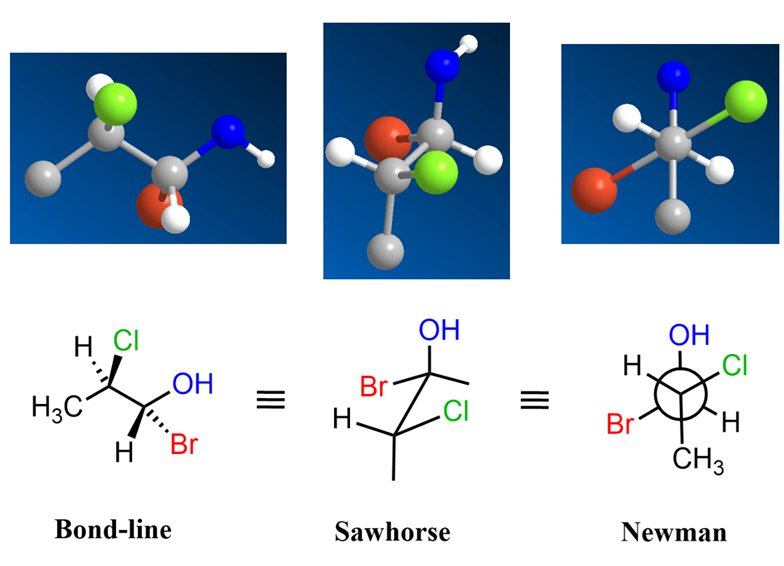
Fischer Projections:
Introduced by Emil Fischer for sugars and amino acids, a Fischer projection is a 2D formula that depicts a chiral carbon as the intersection of horizontal and vertical lines. By convention, horizontal lines represent bonds coming out of the plane (toward the viewer) and vertical lines represent bonds going into the plane (away from the viewer). The Fischer projection is especially useful for molecules with multiple contiguous stereocenters (e.g. sugars) because it lays them out in a clear linear fashion without perspective drawings. An important rule is that rotating a Fischer projection 90° in the plane of the page does not represent the same stereochemistry (it essentially invalidates the projection), whereas rotating by 180° is acceptable. Fischer projections preserve the absolute configuration information of chiral centers in a compact form. (Read more @ https://chiralpedia.com/blog/fischer-projection-hassle-free-way-to-depict-a-stereoformula-in-2d/)
Newman Projections:
While not specifically for denoting chirality, Newman projections are invaluable for visualizing conformations – the rotation about single bonds. In a Newman projection, one looks straight down a bond axis. The front atom is depicted as a dot, and the back atom as a circle; bonds radiating from each are shown as lines at 120° intervals. Newman projections help analyze staggered vs. eclipsed conformations and dihedral angles. For example, looking down the C–C bond of 1,2-dichloroethane, one can draw the different rotamers. Newman projections let chemists identify gauche vs. anti relationships and can show how even achiral molecules may adopt chiral conformations. They are particularly useful for assessing steric hindrance and torsional strain in drug molecules. According to the typical description, “In a Newman projection, we look lengthwise down a specific bond of interest… depict the ‘front’ atom as a dot and the ‘back’ atom as a larger circle”. For an animation rotating a simple molecule like ethane to show how staggered and eclipsed conformers are represented as Newman projections, reinforcing the link between 3D geometry and the planar projection – view here – (https://youtu.be/jUqb-KD9SuY)
Pharmaceutical Relevance
Why does stereochemistry matter so much in drug molecules? The answer lies in biology: most biological macromolecules (enzymes, receptors, DNA, etc.) are chiral, being made up of chiral building blocks (L-amino acids in proteins, D-sugars in DNA/RNA). As a result, these biomolecules interact differently with one enantiomer of a drug versus the other. Often, only one enantiomer (sometimes termed the “eutomer”) fits the target well and elicits the desired effect, while the other (“distomer”) may be less active or produce different effects. For example, the beta blocker propranolol’s S-enantiomer is a potent β-antagonist, whereas the R-enantiomer is essentially inactive at beta receptors. In extreme cases, one enantiomer can even be harmful, as with thalidomide. (Read more @ https://chiralpedia.com/blog/chiral-pharmacology-the-mirror-image-of-drug-development/)

Regulatory agencies now recognize these differences and often require separate evaluation of each enantiomer in drug development (a concept that will be expanded in later parts). Thus, from the very outset of drug design, chemists pay attention to stereochemistry: selecting the correct enantiomer or diastereomer of a lead compound and devising methods to make it in pure form. This series will delve into all these aspects, beginning with fundamental concepts of chirality, then nomenclature, and onward to advanced topics like stereoselective synthesis, analytical methods, and future directions in stereochemistry. In short, stereochemistry is not just an abstract concept – it is a practical factor that can determine a drug’s efficacy, safety, and regulatory approval.
Summary (Part 1)
Stereochemistry deals with 3D molecular structure; chirality refers to handedness of molecules (non-superimposable mirror images).
– Chiral molecules (like many drug molecules) have enantiomers which may behave differently biologically; achiral molecules do not exhibit such handedness.
– Louis Pasteur’s separation of tartaric acid enantiomers in 1848 was a landmark in identifying molecular chirality, explaining optical activity.
– Common representations: wedge-dash (3D perspective in 2D), Fischer projections (2D formulas for stereocenters, especially in sugars/amino acids), and Newman projections (viewing conformations down a bond).
– Stereochemistry is crucial in pharmaceuticals: enantiomers can differ in pharmacological activity and safety. This importance drives the need to study, control, and properly represent stereochemistry throughout drug discovery and development.
Suggested Reading
Eliel, E. L., & Wilen, S. H. (1994). Stereochemistry of Organic Compounds. (Introduction chapters provide a thorough grounding in chirality and historical context.)
Pasteur, L. (1848). Comptes Rendus, 26, 535–538. (English translation available: Pasteur’s original account of tartaric acid crystal separation – a classic read in stereochemistry history.)
IUPAC Gold Book – “Chirality” and “Chiral Centre” definitions. (Authoritative definitions and terminology recommendations.)
Ernest L. Eliel, Samuel H. Wilen, Stereochemistry of Organic Compounds, 1994. ISBN: 978-0-471-01670-0 Wileyhttps://www.wiley.com/en-in/Stereochemistry+of+Organic+Compounds-p-9780471016700
Alan Bassindale, The Third Dimension in Organic Chemistry, 1984. ISBN – 0783732163, 9780783732169.
Jerry March, Advanced Organic Chemistry: Reactions, Mechanisms and Structure, John Wiley & Sons Inc., New York, 2006.
Bernard Testa, Principles of organic stereochemistry, Marcel Dekker Inc., New York, 1979.
https://chiralpedia.com/blog/fischer-projection-hassle-free-way-to-depict-a-stereoformula-in-2d/
FDA Guidance for Industry (1992). Development of New Stereoisomeric Drugs. (Background section provides context on why chirality matters in pharmaceuticals.)
https://en.wikipedia.org/wiki/Chiral_drugs
https://www.pharmabiz.com/NewsDetails.aspx?aid=162712&sid=9
Morrison, J. D., & Mosher, H. S. (1971). Asymmetric Organic Reactions. (Historical perspective on early asymmetric synthesis and resolution methods.)