Levodopa, L-Dopa, belong to a class of medications called dopamine agonists. L-Dopa is used to increase dopamine concentrations in the treatment of Parkinson’s disease. Most commonly, clinicians use levodopa as a dopamine replacement agent for the treatment of Parkinson disease. L-dopa is the precursor to dopamine and crosses the blood-brain barrier to increase dopamine neurotransmission.
Chirality and Biological activity
L-dopa, the (S)-enantiomer, is a chiral drug with one stereogenic center. It exists as a pair of enantiomers. The initial use of racemic dopa for the treatment of Parkinson’s disease resulted in a number of adverse effects viz. nausea, vomiting, anorexia, involuntary movements and granulocytopenia. The use of L-dopa resulted in reducing the required dose, and adverse effects. The pharmacological activity resides in the (S)-enantiomer where as the (R)-version harbors adverse effects.
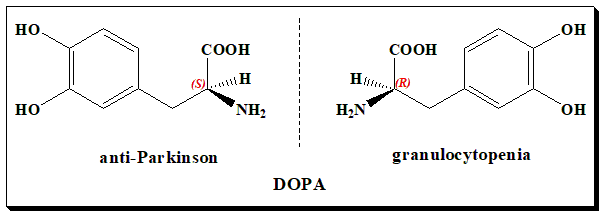
Hence, L-dopa is marketed as a unichiral drug due to serious side-effect, granulocytopenia, attributable to the D-isomer.
Category
Dopamine agonist, Antiparkinson drug
Nomenclature
3,4-dihydroxy-L-phenylalanine
Exercise
Understand the nomenclature and stereo-descriptors employed
References
Chiral drugs. Wikipedia, Wikipedia Foundation, 05/09/2022. https://en.wikipedia.org/wiki/Chiral_drugs and references therein
Hassan Y. Aboul-Enein, Irving W. Wainer, The Impact of Stereochemistry on Drug Development and Use, John Wiley & Sons, New York, 1997. ISBN: 978-0-471-59644-8
https://www.researchgate.net/publication/230209353_Toxicology_of_Chiral_Drugs