Levofloxacin is a third generation fluoroquinolone that is widely used in the treatment of mild-to-moderate respiratory and urinary tract infections due to sensitive organisms. Levofloxacin is an antibacterial prescription medicine approved by the U.S. Food and Drug Administration (FDA) for the treatment of certain bacterial infections, such as community-acquired pneumonia, acute worsening of chronic bronchitis, anthrax, urinary tract infections, acute sinus infections, and others.
Chirality and biological activity
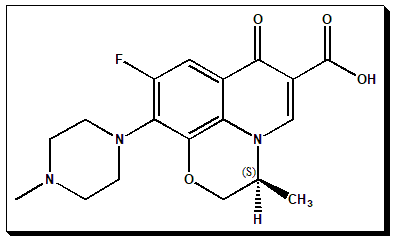
Levofloxacin, the S-enantiomer of the previously marketed racemic antibacterial (±)-Ofloxacin. This is referred to as a chiral switch. (Read more @ my page in Wikipedia – <Chiral switch. Wikipedia, Wikipedia Foundation, 25/07/2022. https://en.wikipedia.org/wiki/Chiral_switch>.) Ofloxacin carries one stereogenic center in the 1,4- oxazine, a heterocyclic ring system, of the molecule and exist as pair of enantiomers. The (S)-version, Levofloxacin, has the desired antibacterial activity and called the eutomer. The (R)-form is pharmacologically inactive and referred to as the distomer. To know more about eutomer and distomer nomenclature read @ <https://chiralpedia.com/blog/chiral-twins-identical-but-not-really/>
The reason for the development of a single-enantiomer of racemic Ofloxacin is that:
- (S)-isomer, Levofloxacin is 8-125 fold greater antibacterial potency.
- Higher water solubility than the R-isomer.
- Also enantiomeric disposition favor single-enantiomer.
Nomenclature
(S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylic acid
Therapeutic category
Anti-bacterial
Exercise
- Number the Levofloxacin heterocyclic ring and understand the nomenclature
- Draw the stereochemical structure of the mirror-image twin of Levofloxacin
References
Joseph Gal in Chirality in drug research. Edited by Eric Francotte, W. Lindner. Weinheim: Wiley-VCH. ISBN 978-3-527-60943-7. Page 3-25, 2006.
Stereochemical Aspects of Drug Action and Disposition,Editors: Eichelbaum, Michel F., Testa, Bernard, Somogyi, Andrew (Eds.) Springer, 2003. https://www.springer.com/gp/book/9783642625756
Wainer, l. W., & Drayer, D. E., Eds. Drug stereochemistry and Pharmacology: Marcel-Dekker, New York,1988.
Chiral drug. Wikipedia, Wikipedia Foundation, 25/07/2022. https://en.wikipedia.org/wiki/Chiral_drugs
Chiral switch. Wikipedia, Wikipedia Foundation, 25/07/2022. https://en.wikipedia.org/wiki/Chiral_switch
Harkishan Singh and V.K. Kapoor. Medicinal chemistry and pharmaceutical chemistry, Vallabh Prakashan, New Delhi, Page 603-06, 2012.