“Where molecules speak, stereochemistry gives them meaning“
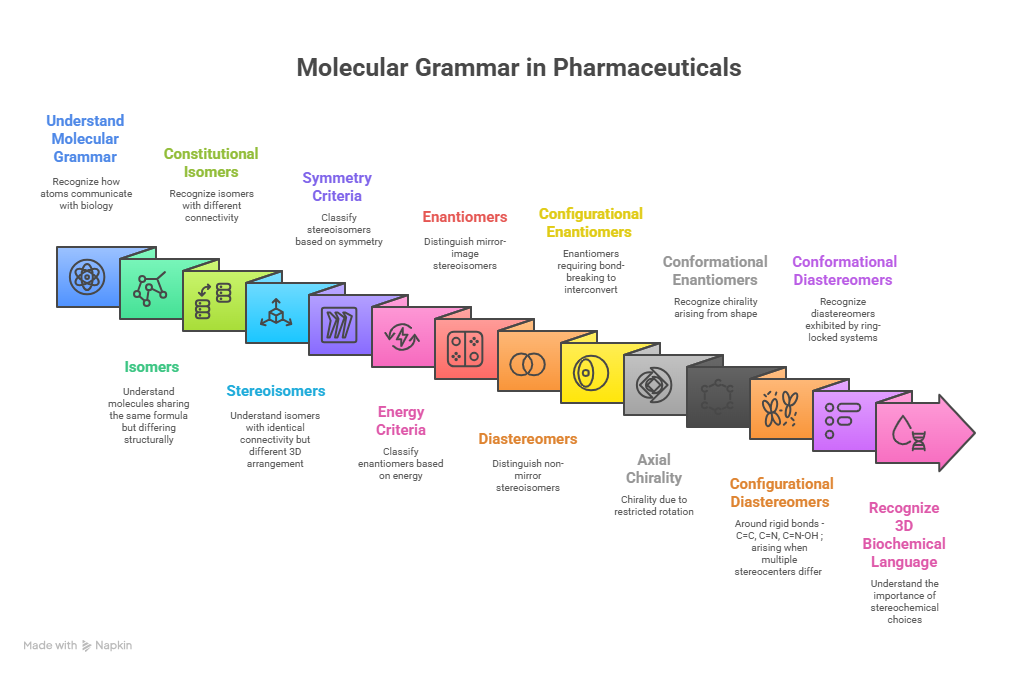
In pharmaceuticals, structure is language — and stereochemistry is its grammar. The way atoms arrange in 3D space shapes how drugs work, how they are regulated, and how safe they are. This Chiralpedia tutorial simplifies the molecular “grammar” behind drug behavior and innovation.
When Structure Speaks
Every drug molecule has a story written in its structure. Two compounds may share the same formula yet act entirely differently due to how atoms connect and orient in space — a principle called isomerism. Within it, stereochemistry adds the three-dimensional “architecture” that determines how molecules fit and function in the body.
In medicine, these shifts are never minor. A mirror-image twist or locked rotation can turn therapy into toxicity or silence biological activity altogether. Understanding these spatial relationships isn’t academic detail — it’s core to designing better drugs and ensuring patient safety.
This tutorial explores how molecular structure, symmetry, and stereogenic elements — from simple chiral centers to axial and conformational systems — and shows how they shape pharmacology, toxicity, and therapeutic precision through real drug examples.
To complement detailed discussion with visual clarity, this article adopts a concept-map format — distilling the key principles of molecular grammar and stereochemical relationships into a coherent visual framework.
Suggestion: Open the mind map in a new tab and zoom out for a clearer, full-view of the concept map.
Every drug molecule speaks a language — its grammar defined by structure, symmetry, and stereochemistry.
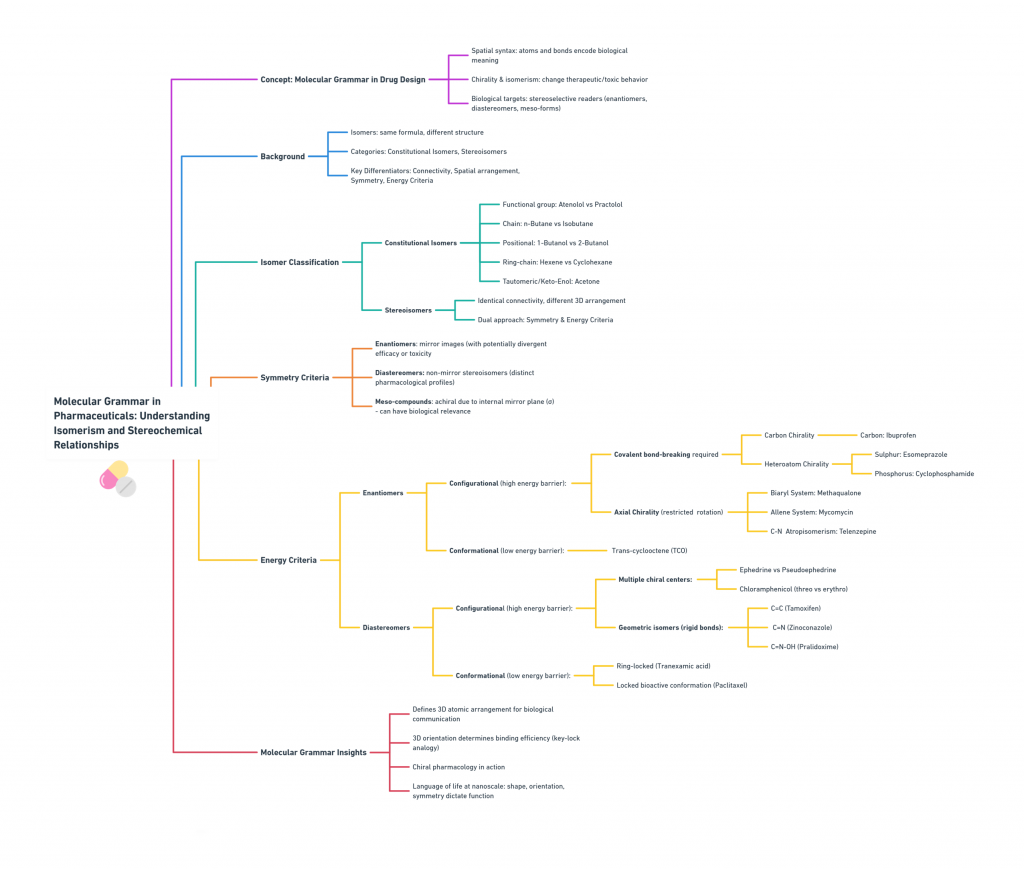
This map decodes how subtle spatial changes transform molecular identity, guiding efficacy, safety, and selectivity in pharmaceuticals. It explores the interplay of isomerism, chirality, symmetry, and energy criteria in shaping a molecule’s therapeutic or toxic potential.
1. The Two Faces of Isomerism: Framework and Form
All isomers share the same molecular formula but differ in one of two fundamental ways:
- Constitutional (Structural) Isomerism: Different atomic connectivity.
- Stereoisomerism: Same connectivity, different 3D orientation.
If molecular formula is vocabulary, then connectivity and geometry are grammar and tone — together shaping how molecules express their biological meaning.
💡 Learning Insight:
Isomerism is chemistry’s way of showing that structure and shape can change meaning even when composition remains constant.
2. Constitutional Isomers: Same Parts, Different Assembly
Definition: Compounds that share the same molecular formula but differ in how atoms are bonded. Small rearrangements can influence solubility, receptor binding, metabolism, and toxicity.
Types: Functional group Isomers (e.g., Atenolol vs Practolol), Chain Isomers (n-Butane vs Isobutane), Positional Isomers (1-Butanol vs 2-Butanol), Ring-chain Isomers (Hexene vs Cyclohexane), Tautomeric, Keto-Enol, Isomers (Acetone).
💠 Structural Element Focus: Chain and functional group connectivity
💊 Pharmaceutical Example: Atenolol vs Practolol
🧩 Stereochemical Type: Structural (not stereochemical) difference
💡 Learning Insight:
Even before 3D orientation, connectivity alone can define therapeutic identity. Changing connectivity alters identity — like rearranging letters into a new word.
3. Stereoisomers: Grammar of Molecular Shape
Stereoisomers share the same connectivity but differ in spatial arrangement. Their geometries arise from stereogenic elements such as:
- Chiral centers (C*, N*, S*, or P* atoms)
- Axes of chirality (e.g., biphenyls, allenes)
- Planes of chirality
- Restricted rotation around C=C, C=N, C=N-OH bonds, etc.
💡 Learning Insight:
The three-dimensional shape of a molecule determines how it interacts with its biological “reader.”
4. Classes of Stereoisomers: Mirror, Mismatch, and Symmetry
4.1 Enantiomers — The Mirror Images
💠 Structural Element Focus: Chiral carbon (C*)
💊 Pharmaceutical Example: Ibuprofen — one chiral carbon
🧩 Stereochemical Type: Enantiomeric pair
4.2 Diastereomers — Not Mirror Images
💠 Structural Element Focus: Multiple chiral centers (two or more C*)
💊 Pharmaceutical Example: Threonine — two chiral carbons → four stereoisomers
🧩 Stereochemical Type: Diastereomeric
4.3 Meso-Compounds — Chiral Centers, Yet Achiral
💠 Structural Element Focus: Internal mirror plane (σ) or center of inversion (i)
💊 Pharmaceutical Example: Meso-tartaric acid
🧩 Stereochemical Type: Achiral despite chiral centers
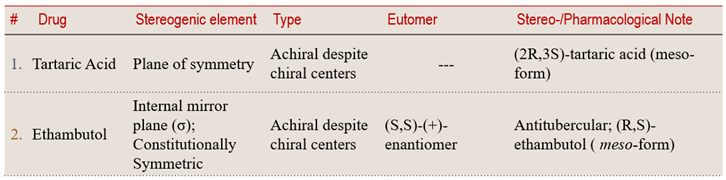
💡 Learning Insight:
Symmetry determines whether a molecule expresses chirality — even when it contains chiral centers.
5. Configurational Enantiomers: Fixed and Faithful Chirality
Configurational enantiomers are non-superimposable mirror images that cannot interconvert without breaking covalent bonds. Their 3D arrangements are rigid and isolable, making them pharmacologically distinct entities.
💡 Learning Insight:
Configurational enantiomers are “locked” chiral forms — each with its own biological story
5.1 Chirality Center – Rigid
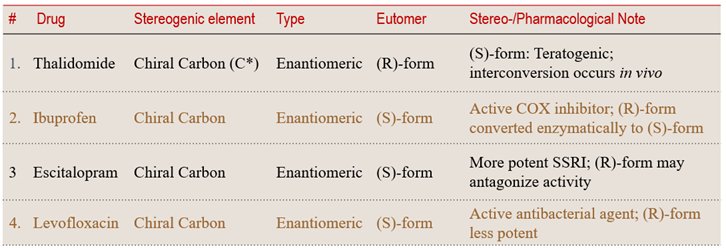
Case 1: Carbon-Based Chirality — The Classical Core
A chirality center (commonly a tetrahedral atom bonded to four different substituents) is the most classical and stable source of molecular chirality. Such enantiomers differ solely in spatial arrangement around this center and cannot interconvert without bond cleavage.
Carbon-based chirality remains the most widely encountered and pharmaceutically significant form of stereochemical differentiation. The following examples illustrate how a single chiral carbon can transform molecular identity, potency, and safety profile.
💠 Structural Element Focus: Chiral carbon (C*)
🧩 Stereochemical Type: Configurational (Enantiomeric)
Case 2: Beyond Carbon — Heteroatom Chirality: Expanding the Chiral Horizon
Chirality is not the exclusive domain of carbon. Heteroatoms such as sulfur, phosphorus, and nitrogen can generate stable, isolable enantiomers with strikingly different pharmacological outcomes. These examples reveal how heteroatom-based chirality broadens stereochemical design in drug molecules.
💠 Structural Element Focus: Chiral heteroatoms (S, P, N)
🧩 Stereochemical Type: Configurational (Heteroatom Enantiomeric)
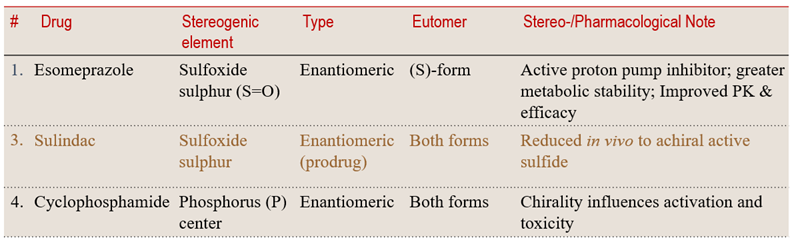
💡 Learning Insight:
Chirality extends beyond carbon — heteroatoms like S and P can create stable enantiomers that dictate a drug’s reactivity, metabolism, and selectivity.
Note:
🧩Chirality Due to Nitrogen: When Inversion Slows the Mirror
Nitrogen atoms are potentially chiral when bonded to three different substituents and possess a lone pair (i.e., are pyramidal).
However, in most amines, rapid inversion (“umbrella inversion”) occurs — typically with an energy barrier of only ~6 kcal/mol — which allows the nitrogen to invert between its mirror forms billions of times per second.
👉 Result: Most simple amines do not exhibit stable chirality because the two configurations interconvert too fast to be isolated. Conditions that create stable Nitrogen chirality Quaternary ammonium centers — no lone pair, inversion impossible; Amidine, imine, or amide nitrogen in sterically hindered or cyclic systems; Bridged or cage systems where nitrogen is part of a rigid bicyclic or polycyclic framework. So when this inversion is restricted — due to ring rigidity, quaternization, or amide formation — nitrogen chirality becomes observable and isolable.
5.2 Axial Chirality — When Rotation Freezes – Chirality from hindered rotation, not atoms.
Some molecules exhibit chirality without a center of chirality When rotation around a bond is hindered by steric or electronic barriers, a molecule can become “locked” in left- or right-handed forms. This phenomenon, known as axial chirality, introduces a fascinating dimension to stereochemical control..
💠 Structural Element Focus: Axial system (C=C=C or aryl–aryl bond)
🧩 Stereochemical Type: Configurational (Axial Enantiomers)
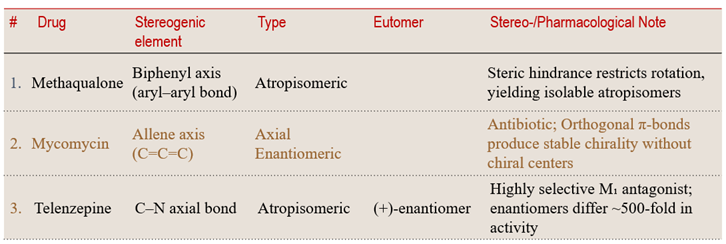
💡 Learning Insight:
When rotation is restricted, geometry becomes destiny — chirality can arise from spatial constraint alone.
6. Conformational Enantiomers: Chirality Through Shape
Some molecules become chiral not because of a traditional stereocenter, but because their 3D folded shape (often helical or twisted) prevents superimposition with its mirror image. In these systems, chirality arises from conformation, not from a static stereogenic atom or axis.
When molecular folding or helical twisting creates a handed form, the resulting conformers can exist as non-superimposable mirror images. These conformational enantiomers often appear in natural products, macrocycles, and aromatic helices — where shape itself encodes biological recognition.
💠 Structural Element Focus: Folded or helical conformation
🧩 Stereochemical Type: Conformational Enantiomerism
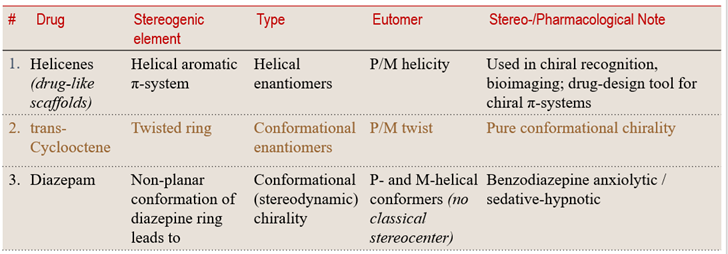
💡 Learning Insight:
Shape can be the stereocenter. When folding or helical geometry prevents mirror-image overlap, a molecule becomes chiral without needing a classical stereogenic atom. True conformational enantiomers are rare in drugs, but foundational in probe chemistry & modern chiral scaffold design.
7. Configurational Diastereomers: – Diastereomers not mirror images, Geometry defines distinct biological behavior.
Diastereomers differ at one or more stereocenters (Interconversion requires bond breaking (fixed configuration at stereocenters) or around rigid bonds such as C=C or C=N (energy barrier to interconversion). These geometric differences often produce distinct pharmacological behaviors — sometimes transforming efficacy, selectivity, or toxicity entirely.
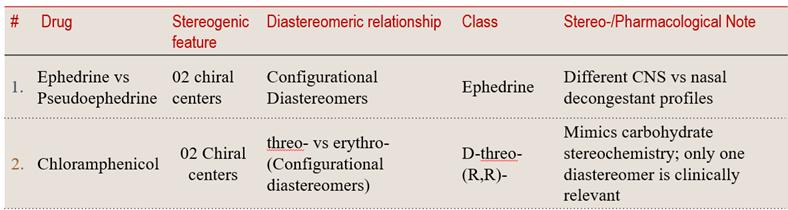
7.1 Diastereomers with two or more chiral centers differ at one or more — but not all — stereocenters
This structural feature is seen in chiral molecules like ephedrine vs pseudoephedrine or the antibiotic Chloramphenicol, where subtle stereochemical changes create clinically distinct profiles.
💠 Structural Element Focus: two or more chiral centers
🧩 Stereochemical Type: Configurational Diastereomers
7.2 Diastereomers can arise from restricted rotation around rigid bonds
These are exhibited by molecules with C=C or C=N bonds, leading to distinct stereoisomers with energy barriers to interconversion.
💠 Structural Element Focus: Restricted rotation (C=C or C=N bond)
🧩 Stereochemical Type: Configurational (Geometric) Diastereomers
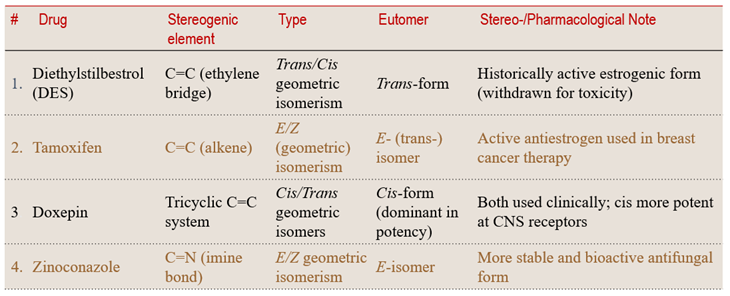
💡 Learning Insight:
Geometric rigidity — whether from a C=C double bond or a C=N imine — can lock molecules into discrete configurations that behave like separate drugs, each with its own pharmacological identity.
7.3 Oxime Drugs under Configurational Diastereomers
Oxime-containing compounds exhibit C=N double-bond isomerism (E/Z or syn/anti). These are diastereomeric relationships, since they are non-mirror-image configurational isomers.
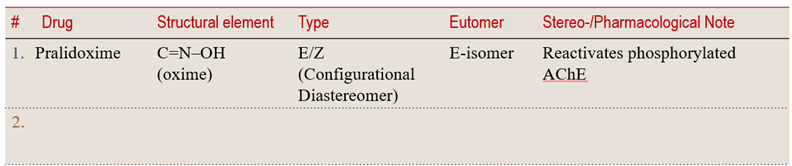
💡 Learning Insight:
Oximes illustrate C=N–based configurational diastereomerism.
The E/Z (or syn/anti) orientation across the C=N bond defines their pharmacological profile — influencing stability, reactivity, and bioactivation, especially in oxime prodrugs and β-lactam antibiotics.
8. Conformational Diastereomers — Dynamic Origins, Rigid Outcomes
These arise when flexible molecules become locked by steric hindrance, ring strain, or internal H-bonding into distinct, stable conformations. Flexible molecules that “freeze” into biologically distinct shapes.
💠 Structural Element Focus: Conformational locking in cyclic or macrocyclic frameworks
🧩 Stereochemical Type: Conformational Diastereomerism
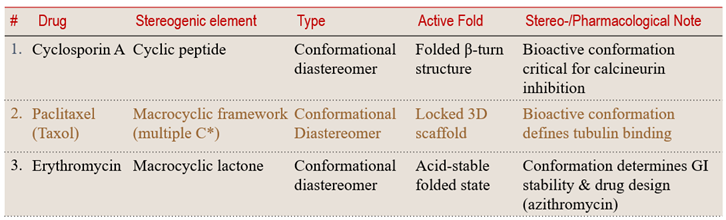
8.1 Dynamic to Rigid: Macrocyclic and Peptide Systems – Conformational control is a design tool for potency, selectivity, and stability.
Macrocycles and cyclic peptides can adopt multiple dynamic conformations, yet only one often aligns perfectly with the biological target. When internal H-bonding or steric pressure locks a fold, a stable conformational diastereomer emerges.
8.2 Conformational Locking in Small Rings and Drug Scaffolds
Small, strained ring systems — or rigid fused motifs — may exist in multiple conformational states but preferentially adopt one under physiological conditions. These locked arrangements behave like distinct stereoisomers.
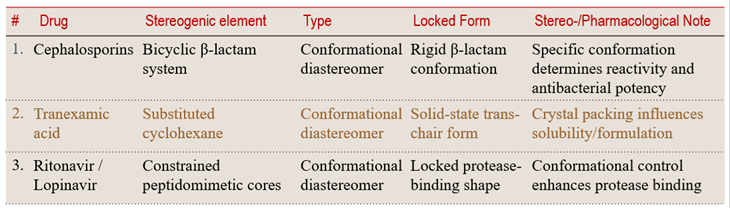
💡 Learning Insight:
Conformations may be dynamic in origin, but once constrained, they act as rigid structural identities — influencing both biological activity and formulation behavior.
9. The Molecular Grammar of Medicines: Decoding the Syntax of Structure
The study of isomerism reveals that molecular identity is not solely about composition, but about arrangement — the way atoms claim space, express symmetry, and interact with biological targets. In the pharmaceutical world, these structural subtleties become the difference between activity and inactivity, safety and toxicity, efficacy and failure.
Every enantiomer, diastereomer, or conformational variant carries its own message — one encoded in the molecular grammar of shape and symmetry. Whether the stereochemical feature arises from a chiral carbon, a sulfoxide sulfur, a phosphorus center, or an axial bond system, its spatial consequence determines how a drug behaves in the body. Together, they form the molecular grammar that connects structure to biological function.
For medicinal chemists, pharmacologists, and students of molecular science, understanding this grammar is essential. It transforms stereochemistry from an abstract idea into a predictive tool — one that explains why two identical formulas can lead to profoundly different pharmacological destinies.
In the end, every drug is a story written in structure — and isomerism is its syntax, defining how chemistry gives meaning to medicine.
10. 💬 Final Takeaway
Mastering isomerism means mastering the language of molecular form.
Every twist, plane, and mirror defines how chemistry gives meaning to medicine — and how one formula can tell two very different therapeutic stories.
Stereochemistry is not decoration — it’s destiny. In pharmaceuticals, the 3D script of atoms dictates how molecules speak to biology.
Mastering this molecular grammar empowers:
- Smarter medicinal chemistry
- Safer and more selective therapies
- Stronger IP landscapes
- Useful AI-driven molecular design
References & Further Reading
Textbooks & Monographs
Eliel, E. L., Wilen, S. H., & Mander, L. N. (1994). Stereochemistry of organic compounds. Wiley.
Patrick, G. (2021). An introduction to medicinal chemistry (7th ed.). Oxford University Press.
Silverman, R. B., & Holladay, M. W. (2014). The organic chemistry of drug design and drug action (3rd ed.). Academic Press.
Key Review Articles
Caldwell J. The importance of stereochemistry in drug action and disposition. J Clin Pharmacol. 1992 Oct;32(10):925-9. doi: 10.1002/j.1552-4604.1992.tb04640.x
LaPlante, S. R., Edwards, P. J., Fader, L. D., Jakalian, A., & Hucke, O. (2011). Revealing atropisomer axial chirality in drug discovery. ChemMedChem, 6(3), 505–513. https://doi.org/10.1002/cmdc.201000485
Nguyen LA, He H, Pham-Huy C. Chiral drugs: an overview. Int J Biomed Sci. 2006 Jun;2(2):85-100. PMID: 23674971; PMCID: PMC3614593.
Hui Yang, Hong-Xia Feng, Ling Zhou, Asymmetric Synthesis of Helicenes from Centrally Chiral Precursors, Eur. J. Org. Chem., July, 2024. https://doi.org/10.1002/ejoc.202400671
Yun Shen, Chuan-Feng Chen, Helicenes: Synthesis and Applications, Chem. Rev. 2012, 112, 3, 1463–1535. https://doi.org/10.1021/cr200087r
Smith SW. Chiral toxicology: it’s the same thing…only different. Toxicol Sci. 2009 Jul;110(1):4-30. doi: 10.1093/toxsci/kfp097. Epub 2009 May 4. PMID: 19414517.
Bioorthogonal Chemistry & Conformational Chirality
Blackman, M. L., Royzen, M., & Fox, J. M. (2008). Tetrazine ligation: Fast bioorthogonal reactions for bioconjugation. Journal of the American Chemical Society, 130(41), 13518–13519. https://doi.org/10.1021/ja805847d
Prescher, J., Bertozzi, C. Chemistry in living systems. Nat Chem Biol 1, 13–21 (2005). https://doi.org/10.1038/nchembio0605-13; http://www.nature.com/naturechemicalbiology/
Regulatory & Guidelines
FDA’s policy statement for the development of new stereoisomeric drugs. Chirality. 1992;4(5):338-40. doi: 10.1002/chir.530040513. PMID: 1354468.
International Council for Harmonisation. (1999). ICH Q6A: Specifications—Test procedures and acceptance criteria for new drug substances and new drug products: Chemical substances. ICH Secretariat.
European Medicines Agency. (2001). Note for guidance on the investigation of chiral active substances. EMA.
Community Note:
Chiralpedia strives to make stereochemistry understandable, relevant, and useful for learners and professionals alike. We welcome your insights — if you spot an error or wish to suggest an enhancement, please share it in the feedback section.
Chiralpedia continues to evolve through the curiosity and contributions of its readers.