“Chirality isn’t hidden — we just stopped looking closely enough”
The Unseen Journey After the Dose:
💊 When a patient swallows a drug, the journey is far from over. Sometimes, the real chiral story begins after the dose. The drug molecule meets a series of enzymes — oxidases, reductases, transferases — each capable of transforming it into one or more metabolites. We often assume these are simply inactive breakdown products, but chemistry rarely plays it that straight.
In the chiral world, metabolism can do something extraordinary — it can invert, create, or amplify chirality. What starts as a single enantiomer may emerge as multiple stereoisomeric metabolites, each with its own pharmacological identity. Yet, most drug development programs and even regulatory documents remain silent on this stereochemical evolution.
This silence represents a metabolic blind spot — one that can affect everything from drug safety to efficacy, and yet, remains overlooked in pharmacological discourse.
How Metabolism Makes New Chirality
Drug metabolism doesn’t merely deactivate molecules; it remodels them.
- Phase I reactions (oxidation, reduction, hydrolysis) can introduce new chiral centers, for instance, by hydroxylating prochiral carbons.
- Phase II reactions (such as conjugation with glucuronic acid or sulfate) may also yield diastereomeric conjugates.
These transformations are stereoselective, often governed by the chiral nature of metabolic enzymes themselves. Cytochrome P450 isoforms, for example, display enantioselectivity — preferring one enantiomer over another, or acting differently on each.
As a result, the body may process the left-handed and right-handed versions of a chiral molecule differently, yielding distinct pharmacokinetic and pharmacodynamic outcomes. Selected live examples are presented below for illustration.
The Case of Ibuprofen — A Metabolic Twist in Action
Among over-the-counter drugs, ibuprofen is perhaps the best-known case of chiral metabolism. It is marketed as a racemate, but only the S-(+)-enantiomer inhibits cyclooxygenase (COX) enzymes and relieves pain.
Here’s the twist: in the body, the supposedly inactive R-(-)-enantiomer undergoes metabolic inversion — converted enzymatically into the active S-form. This chiral inversion is catalyzed by acyl-CoA synthetase and 2-arylpropionyl-CoA epimerase, mainly in the liver.
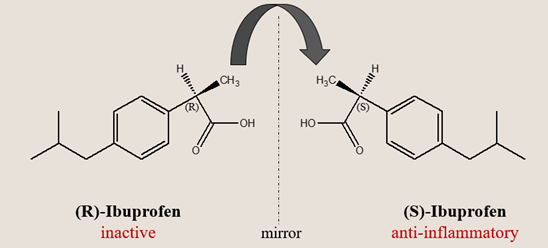
Thus, both enantiomers ultimately contribute to therapeutic activity, but through different routes — one directly, one metabolically. This raises a profound question: when a drug’s efficacy depends on metabolic inversion, should we still treat it as a racemate (1:1 ratio) or a chiral mixture?
Note: A chiral mixture is a broader term that can refer to any combination of chiral molecules, including a racemate (1:1 ratio), an enantioenriched mixture with an excess of one enantiomer.
Thalidomide — The Classic Cautionary Tale, Revisited
No discussion on chiral metabolism can escape thalidomide — the molecule that taught the world how dangerous stereochemical indifference can be.
When first marketed in the 1950s as a racemate, one enantiomer was sedative and antiemetic, while the other was teratogenic. In theory, producing only the “safe” enantiomer should have prevented harm. But metabolism defied that logic.
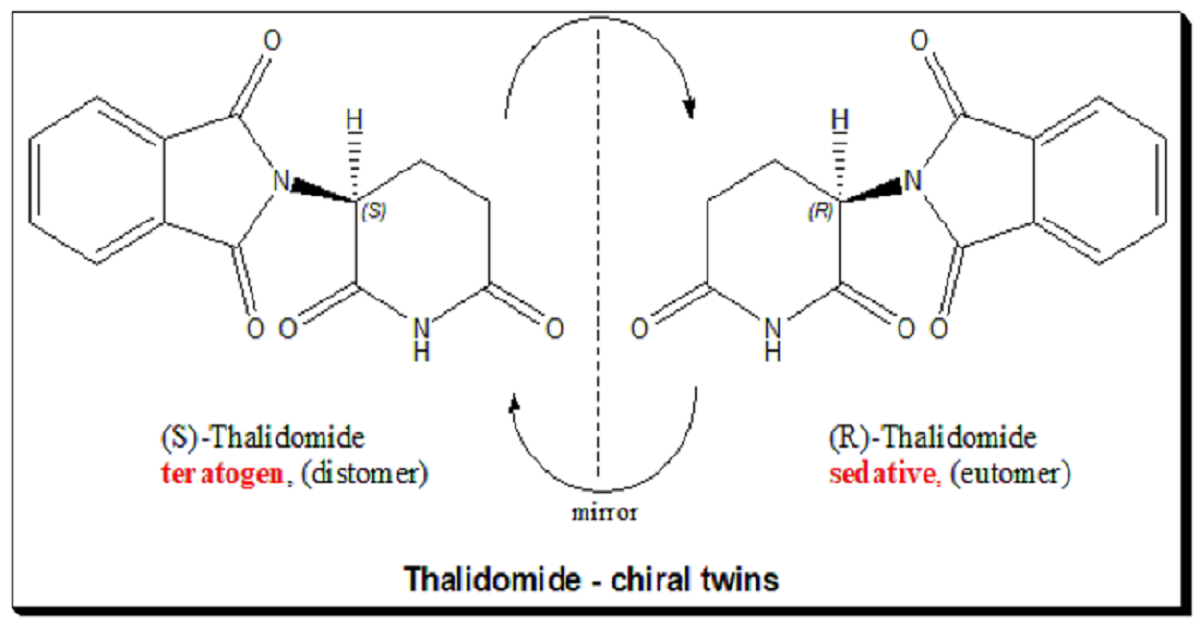
This realization shattered the illusion that a single enantiomer guarantees purity of effect. It also emphasized the metabolic fluidity of chirality — how biological systems can blur the lines chemists try to draw.
The Thalidomide tragedy (see <https://pubmed.ncbi.nlm.nih.gov/21507989/>) led to profound and lasting shifts in toxicity-testing protocols, regulatory oversight, and how chemists view chiral molecules. Today, when it comes to chiral drugs, each enantiomer is treated as a separate chemical entity—almost as though one drug were two distinct therapies.
Propranolol and Citalopram — When Metabolites Rewrite Pharmacology
The story repeats across many therapeutic classes.
- Propranolol, a β-blocker used in cardiac therapy, exhibits enantioselective metabolism: the S-(–)-form is more potent pharmacologically, but both enantiomers produce metabolites with differing receptor affinities and elimination rates.
- Citalopram, an antidepressant, also demonstrates chiral complexity. The S-enantiomer (escitalopram) is the active component, yet the R-form and its metabolites can antagonize the S-form’s serotonin transporter binding — subtly modulating efficacy.
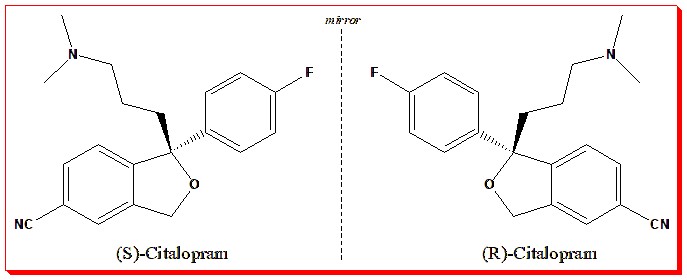
Citalopram is a chiral antidepressant made up of two mirror-image forms, or enantiomers — (S)-citalopram (better known as escitalopram) and (R)-citalopram. Among the two, it’s the (S)-enantiomer that truly drives the drug’s antidepressant power. It acts as a highly selective serotonin reuptake inhibitor (SSRI), enhancing serotonin levels in the brain. The (R)-enantiomer, on the other hand, is about 20 times less potent at blocking serotonin reuptake and can even interfere with the beneficial action of its (S)-counterpart. In other words, one enantiomer heals, while the other hinders.
In both cases, metabolism not only modifies the molecule — it modifies the message. The metabolites can reinforce, compete with, or counteract the parent compound’s action.
Analytical Blindness — The Technical Bottleneck
One reason chirality in metabolites remains challenging lies in analytical limitations. Detecting and quantifying enantiomeric metabolites in biological fluids is a difficult task.
- Chiral chromatography requires highly specialized stationary phases and careful method validation.
- Mass spectrometry alone cannot distinguish enantiomers — it sees only mass, not handedness.
- NMR and circular dichroism can help, but demand pure isolates and sophisticated interpretation.
Because of these challenges, many pharmacokinetic studies still report “total metabolite” concentrations without resolving enantiomeric forms. What’s lost in that averaging is often pharmacologically critical.
The Regulatory Silence
The U.S. FDA’s 1992 “Development of New Stereoisomeric Drugs” guidance was a milestone, urging that enantiomers be characterized separately for pharmacology, pharmacokinetics, and toxicology. However, that guidance remains non-binding, and it focuses primarily on the parent drug. There is still no regulatory requirement to study the stereochemistry of metabolites — even though the guidance itself acknowledges that “stereoisomeric metabolites may contribute to drug action or toxicity.”
This creates an uneven landscape: the chirality of the starting material is scrutinized, but the chirality of its metabolic products often slips through unexamined.
Why This Blind Spot Matters
Ignoring chirality at the metabolite level can have serious implications:
- Misjudged potency: Active enantiomeric metabolites can lead to underestimated efficacy.
- Unexpected toxicity: One enantiomeric metabolite may bind unintended receptors or accumulate selectively.
- Variable pharmacokinetics: Stereoselective metabolism can differ among individuals or ethnic groups, affecting dosing precision.
In the era of personalized medicine, such oversights run counter to our goal of precision pharmacotherapy. Understanding metabolic chirality isn’t academic — it’s essential.
A Call for Chiral Literacy
If chemistry teaches us anything, it’s that symmetry hides complexity. In pharmacology, the mirror image of a molecule can mean the mirror image of an outcome. Chiralpedia’s mission is to illuminate these hidden corners — where regulatory documents, AI models, or metabolic pathways lose sight of stereochemistry. The overlooked chirality of metabolites is one such corner, rich with both scientific challenge and clinical consequence.
To move forward, we must integrate enantioselective metabolic studies into routine drug development, enhance analytical methods for metabolite chirality, and most importantly, foster chiral literacy among students and researchers alike.
After all, the next time we design a “single-enantiomer” drug, we should ask: What will the body make of it — and what chiral stories will its metabolites tell?
References and Further Reading
Hutt, A. J., & Caldwell, J. (1983). The Metabolic Chiral Inversion of 2-Arylpropionic Acids — A Novel Route with Pharmacological Consequences. Journal of Pharmacology and Experimental Therapeutics, 224(3), 658–664. — Classic paper detailing metabolic inversion of (R)- to (S)-ibuprofen and other NSAIDs — the canonical example of in vivo chirality switch.
Aboul-Enein HY, Kannappan V, Kanthiah S. Chiral Inversion of Pharmaceutical Drugs – Mini Review. Comb Chem High Throughput Screen. 2023;26(15):2577-2582. doi: 10.2174/1386207326666230102123655
“Chiral inversion.” Wikipedia: The Free Encyclopedia. Wikimedia Foundation, Inc. Accessed [24-10-2025]. https://en.wikipedia.org/wiki/Chiral_inversion
Caldwell, J. (1995). Pharmacological and Toxicological Implications of the Stereochemistry of Drug Metabolism. Pharmacology & Therapeutics, 45(3), 343–395. — A comprehensive review of stereoselective oxidation, reduction, and conjugation reactions.
Ariëns, E. J. (1984). Stereochemistry, a Basis for Sophisticated Nonsense in Pharmacokinetics and Clinical Pharmacology. European Journal of Clinical Pharmacology, 26(6), 663–668. — A witty yet insightful reminder of why neglecting stereochemistry distorts pharmacological interpretation.
Wsol, V., Skalova, L., & Szotakova, B. (2004). Enantioselectivity of Drug Metabolizing Enzymes. Current Drug Metabolism, 5(6), 517–533. — Discusses enantioselective metabolism by cytochrome P450s, esterases, and reductases across major drug classes.
Williams, K. M., et al. (1999). Stereoselective Formation and Clearance of Omeprazole Metabolites in Humans. Clinical Pharmacology & Therapeutics, 66(5), 509–517. — Demonstrates how P450 polymorphisms and chirality together modulate therapeutic outcome.
Kishimoto, W., et al. (2000). Stereoselective Oxidation of Propranolol by Human CYP2D6 and CYP1A2. Drug Metabolism and Disposition, 28(7), 848–853. — A model case of enantioselective metabolism leading to pharmacodynamic asymmetry.
Jacquot C, et al., (2007). Escitalopram et Citalopram: le rôle inattendu de l’énantiomère R [Escitalopram and citalopram: the unexpected role of the R-enantiomer]. Encephale. 33(2):179-87. French. doi: 10.1016/s0013-7006(07)91548-1.
Tamura, H. O., et al. (1993). Stereoselective Metabolism of Mefloquine and Its Implications for Antimalarial Efficacy and Toxicity. Drug Metabolism Reviews, 25(2–3), 219–244. — An instructive example of metabolic chirality influencing drug safety and duration.
Zhang, D., & Zhu, M. (2019). Drug Metabolism in Drug Design and Development (2nd Ed.). Wiley. — Provides an integrated view of how stereoselective biotransformation shapes pharmacokinetics and toxicokinetics.
Darton, C. D., & Yu, A. M. (2023). Chiral Drug Metabolism: The Overlooked Dimension in Pharmacogenomics. Frontiers in Pharmacology, 14, 1130782. — Recent discussion linking chirality to precision medicine and interindividual variability.
Park, B. K., & Kitteringham, N. R. (1994). Drug Metabolism and Drug Toxicity — The Role of Reactive Intermediates. Chemical Research in Toxicology, 7(3), 307–319. — Many reactive metabolites are chiral; this paper connects metabolic asymmetry to idiosyncratic toxicity.
Liu, H., et al. (2015). Chiral Metabolomics: Analytical Advances and Biological Implications. TrAC Trends in Analytical Chemistry, 74, 141–150. — Modern analytical methods for detecting enantiomeric metabolites in biological systems.
Fasinu, P. S., et al. (2019). Enantioselective Metabolism of Chiral Drugs and Its Implications for Drug Safety and Efficacy. Drug Metabolism Reviews, 51(3), 308–323. — Bridges stereoselective biotransformation and clinical pharmacology.
FDA (1992). Development of New Stereoisomeric Drugs: Guidance for Industry. — Notes the importance of metabolite stereochemistry in safety evaluation.
EMA (2011). Guideline on the Investigation of Drug Interactions. — References stereoselective metabolic pathways in drug–drug interaction assessment.
Kannappan, V. (2025). When Labels Miss the Twist: Chirality and the Enantiomer Gap. Chiralpedia Blog. — Explores the regulatory blind spot complementing the metabolic one.
ChiralMetDB (2024). Database of Stereoselective Metabolic Reactions and Chiral Metabolites. https://chiralmetdb.org — A growing resource cataloging chiral metabolites and their enzymatic origins.
Human Metabolome Database (HMDB). https://hmdb.ca — Provides stereochemical annotation for thousands of endogenous and xenobiotic metabolites.